A robust pooled testing approach to expand COVID-19 screening capacity
- PMID: 33556129
- PMCID: PMC7870054
- DOI: 10.1371/journal.pone.0246285
A robust pooled testing approach to expand COVID-19 screening capacity
Abstract
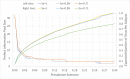
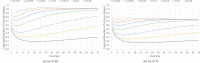
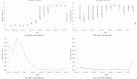
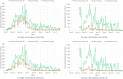
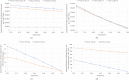
Limited testing capacity for COVID-19 has hampered the pandemic response. Pooling is a testing method wherein samples from specimens (e.g., swabs) from multiple subjects are combined into a pool and screened with a single test. If the pool tests positive, then new samples from the collected specimens are individually tested, while if the pool tests negative, the subjects are classified as negative for the disease. Pooling can substantially expand COVID-19 testing capacity and throughput, without requiring additional resources. We develop a mathematical model to determine the best pool size for different risk groups, based on each group's estimated COVID-19 prevalence. Our approach takes into consideration the sensitivity and specificity of the test, and a dynamic and uncertain prevalence, and provides a robust pool size for each group. For practical relevance, we also develop a companion COVID-19 pooling design tool (through a spread sheet). To demonstrate the potential value of pooling, we study COVID-19 screening using testing data from Iceland for the period, February-28-2020 to June-14-2020, for subjects stratified into high- and low-risk groups. We implement the robust pooling strategy within a sequential framework, which updates pool sizes each week, for each risk group, based on prior week's testing data. Robust pooling reduces the number of tests, over individual testing, by 88.5% to 90.2%, and 54.2% to 61.9%, respectively, for the low-risk and high-risk groups (based on test sensitivity values in the range [0.71, 0.98] as reported in the literature). This results in much shorter times, on average, to get the test results compared to individual testing (due to the higher testing throughput), and also allows for expanded screening to cover more individuals. Thus, robust pooling can potentially be a valuable strategy for COVID-19 screening.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Global cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University 2020 [cited 2020]. Available from: https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd4029942....
-
- WHO Director-General’s opening remarks at the media briefing on COVID-19-16 March 2020: World Health Organization; 2020. [cited 2020]. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-re....
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

