Microglia and Central Nervous System-Associated Macrophages-From Origin to Disease Modulation
- PMID: 33556248
- PMCID: PMC8085109
- DOI: 10.1146/annurev-immunol-093019-110159
Microglia and Central Nervous System-Associated Macrophages-From Origin to Disease Modulation
Abstract
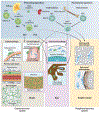
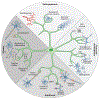
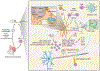
The immune system of the central nervous system (CNS) consists primarily of innate immune cells. These are highly specialized macrophages found either in the parenchyma, called microglia, or at the CNS interfaces, such as leptomeningeal, perivascular, and choroid plexus macrophages. While they were primarily thought of as phagocytes, their function extends well beyond simple removal of cell debris during development and diseases. Brain-resident innate immune cells were found to be plastic, long-lived, and host to an outstanding number of risk genes for multiple pathologies. As a result, they are now considered the most suitable targets for modulating CNS diseases. Additionally, recent single-cell technologies enhanced our molecular understanding of their origins, fates, interactomes, and functional cell statesduring health and perturbation. Here, we review the current state of our understanding and challenges of the myeloid cell biology in the CNS and treatment options for related diseases.
Keywords: development; fate mapping; macrophages; microglia; multiple sclerosis; single-cell profiling.
Figures

References
-
- Ginhoux F, Guilliams M 2016. Tissue-resident macrophage ontogeny and homeostasis. Immunity 44(3):439–49 - PubMed
-
- Kierdorf K, Masuda T, Jordao MJC, Prinz M 2019. Macrophages at CNS interfaces: ontogeny and function in health and disease. Nat. Rev. Neurosci. 20(9): 547–62 - PubMed
-
- Prinz M, Erny D, Hagemeyer N 2017. Ontogeny and homeostasis of CNS myeloid cells. Nat. Immunol. 18(4): 3 85–92 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

