Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial
- PMID: 33556319
- PMCID: PMC7906707
- DOI: 10.1016/S2213-2600(20)30566-X
Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial
Abstract
Background: To date, only monoclonal antibodies have been shown to be effective for outpatients with COVID-19. Interferon lambda-1 is a type III interferon involved in innate antiviral responses with activity against respiratory pathogens. We aimed to investigate the safety and efficacy of peginterferon lambda in the treatment of outpatients with mild-to-moderate COVID-19.
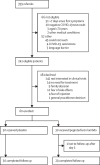
Methods: In this double-blind, placebo-controlled trial, outpatients with laboratory-confirmed COVID-19 were randomly assigned to a single subcutaneous injection of peginterferon lambda 180 μg or placebo within 7 days of symptom onset or first positive swab if asymptomatic. Participants were randomly assigned (1:1) using a computer-generated randomisation list created with a randomisation schedule in blocks of four. At the time of administration, study nurses received a sealed opaque envelope with the treatment allocation number. The primary endpoint was the proportion of patients who were negative for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA on day 7 after the injection, analysed by a χ2 test following an intention-to-treat principle. Prespecified analysis of the primary endpoint, adjusted for baseline viral load, using bivariate logistic regression was done. The trial is now complete. This trial is registered with ClinicalTrials.gov, NCT04354259.
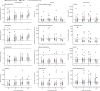
Findings: Between May 18, and Sept 4, 2020, we recruited 30 patients per group. The decline in SARS-CoV-2 RNA was greater in those treated with peginterferon lambda than placebo from day 3 onwards, with a difference of 2·42 log copies per mL at day 7 (p=0·0041). By day 7, 24 (80%) participants in the peginterferon lambda group had an undetectable viral load, compared with 19 (63%) in the placebo group (p=0·15). After controlling for baseline viral load, patients in the peginterferon lambda group were more likely to have undetectable virus by day 7 than were those in the placebo group (odds ratio [OR] 4·12 [95% CI 1·15-16·73; p=0·029). Of those with baseline viral load above 106 copies per mL, 15 (79%) of 19 patients in the peginterferon lambda group had undetectable virus on day 7, compared with six (38%) of 16 in the placebo group (OR 6·25 [95% CI 1·49-31·06]; p=0·012). Peginterferon lambda was well tolerated, and adverse events were similar between groups with mild and transient aminotransferase, concentration increases more frequently observed in the peginterferon lambda group. Two individuals met the threshold of grade 3 increase, one in each group, and no other grade 3 or 4 laboratory adverse events were reported.
Interpretation: Peginterferon lambda accelerated viral decline in outpatients with COVID-19, increasing the proportion of patients with viral clearance by day 7, particularly in those with high baseline viral load. Peginterferon lambda has potential to prevent clinical deterioration and shorten duration of viral shedding.
Funding: The Toronto COVID-19 Action Initiative, University of Toronto, and the Ontario First COVID-19 Rapid Research Fund, Toronto General & Western Hospital Foundation.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures



References
-
- Aoki FY, Macleod MD, Paggiaro P, et al. Early administration of oral oseltamivir increases the benefits of influenza treatment. J Antimicrob Chemother. 2003;51:123–129. - PubMed
-
- Carrat F, Duval X, Tubach F, et al. Effect of oseltamivir, zanamivir or oseltamivir-zanamivir combination treatments on transmission of influenza in households. Antivir Ther. 2012;17:1085–1090. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

