Overview of Humira® Biosimilars: Current European Landscape and Future Implications
- PMID: 33556387
- PMCID: PMC8014989
- DOI: 10.1016/j.xphs.2021.02.003
Overview of Humira® Biosimilars: Current European Landscape and Future Implications
Abstract
Humira® (adalimumab) by AbbVie has been the top-selling biologic drug product for the last few years - reaching nearly $20 billion in annual sales in 2018. Upon the October 2018 release of four adalimumab biosimilars into the European market, those sales began to shrink. By the end of 2019, the annual sales of Humira®, albeit still high, dipped closer to $19 billion as nearly 35% of European patients had been switched from Humira® to a biosimilar. Diminishing sales are expected to continue as the adoption of adalimumab biosimilars increases in Europe and Humira®'s patent protection is lost in the United States come 2023. In this review we discuss how impactful the availability of biosimilars has been to the European adalimumab market approximately two years after their release. We further analyze the marketed biosimilars with regards to differences in their formulation, delivery devices, biological activity, physicochemical properties, clinical trials data, and current financial foothold. More importantly, though, we highlight how "similar" these biosimilars are to Humira®. In doing so, we seek to educate the public on what they may be able to expect once adalimumab biosimilars enter the United States market in 2023.
Keywords: Antibody drug(s); Biosimilar(s); Clinical trial(s); Formulation; Physicochemical properties.
Copyright © 2021 American Pharmacists Association®. Published by Elsevier Inc. All rights reserved.
Figures
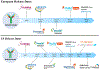
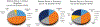

References
-
- Mikulic M Revenue of AbbVie’s Humira 2011–2019. Statista. Published February 28, 2020. Accessed May 5, 2020. https://www.statista.com/statistics/318206/revenue-of-humira/
-
- FDA CDER. HIGHLIGHTS OF PRESCRIBING INFORMATION-HUMIRA.; 2002. Accessed June 4, 2019. www.fda.gov/medwatch
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

