Development and Effects of Influenza Antiviral Drugs
- PMID: 33557246
- PMCID: PMC7913928
- DOI: 10.3390/molecules26040810
Development and Effects of Influenza Antiviral Drugs
Abstract
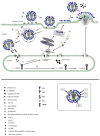
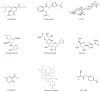
Influenza virus is a highly contagious zoonotic respiratory disease that causes seasonal outbreaks each year and unpredictable pandemics occasionally with high morbidity and mortality rates, posing a great threat to public health worldwide. Besides the limited effect of vaccines, the problem is exacerbated by the lack of drugs with strong antiviral activity against all flu strains. Currently, there are two classes of antiviral drugs available that are chemosynthetic and approved against influenza A virus for prophylactic and therapeutic treatment, but the appearance of drug-resistant virus strains is a serious issue that strikes at the core of influenza control. There is therefore an urgent need to develop new antiviral drugs. Many reports have shown that the development of novel bioactive plant extracts and microbial extracts has significant advantages in influenza treatment. This paper comprehensively reviews the development and effects of chemosynthetic drugs, plant extracts, and microbial extracts with influenza antiviral activity, hoping to provide some references for novel antiviral drug design and promising alternative candidates for further anti-influenza drug development.
Keywords: chemosynthetic drugs; drug resistance; influenza virus; microbial metabolites; plant extracts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Antiviral Activity of Aspalathus linearis against Human Influenza Virus.Nat Prod Commun. 2017 Apr;12(4):599-602. Nat Prod Commun. 2017. PMID: 30520604
-
Perspective of Use of Antiviral Peptides against Influenza Virus.Viruses. 2015 Oct 20;7(10):5428-42. doi: 10.3390/v7102883. Viruses. 2015. PMID: 26492266 Free PMC article. Review.
-
Polyunsaturated fatty acids from Phyllocaulis boraceiensis mucus block the replication of influenza virus.Arch Microbiol. 2018 Aug;200(6):961-970. doi: 10.1007/s00203-018-1507-1. Epub 2018 Apr 3. Arch Microbiol. 2018. PMID: 29616305
-
Anti-Influenza Drug Discovery and Development: Targeting the Virus and Its Host by All Possible Means.Adv Exp Med Biol. 2021;1322:195-218. doi: 10.1007/978-981-16-0267-2_8. Adv Exp Med Biol. 2021. PMID: 34258742
-
Influenza chemotherapy: a review of the present state of art and of new drugs in development.Arch Virol. 2000;145(11):2233-48. doi: 10.1007/s007050070017. Arch Virol. 2000. PMID: 11205114 Review.
Cited by
-
Antiviral effects of Atractyloside A on the influenza B virus (Victoria strain) infection.Front Microbiol. 2023 Jan 10;13:1067725. doi: 10.3389/fmicb.2022.1067725. eCollection 2022. Front Microbiol. 2023. PMID: 36704555 Free PMC article.
-
Myeloid Protease-Activated Receptor-2 Contributes to Influenza A Virus Pathology in Mice.Front Immunol. 2021 Dec 1;12:791017. doi: 10.3389/fimmu.2021.791017. eCollection 2021. Front Immunol. 2021. PMID: 34925374 Free PMC article.
-
Conformation and Membrane Topology of the N-Terminal Ectodomain of Influenza A M2 Protein.Membranes (Basel). 2025 Feb 1;15(2):40. doi: 10.3390/membranes15020040. Membranes (Basel). 2025. PMID: 39997666 Free PMC article.
-
Seleno-Metabolites and Their Precursors: A New Dawn for Several Illnesses?Metabolites. 2022 Sep 16;12(9):874. doi: 10.3390/metabo12090874. Metabolites. 2022. PMID: 36144278 Free PMC article. Review.
-
Antiviral Drugs in Influenza.Int J Environ Res Public Health. 2022 Mar 4;19(5):3018. doi: 10.3390/ijerph19053018. Int J Environ Res Public Health. 2022. PMID: 35270708 Free PMC article. Review.
References
-
- Sharabi S., Drori Y., Micheli M., Friedman N., Orzitzer S., Bassal R., Glatman-Freedman A., Shohat T., Mendelson E., Hindiyeh M., et al. Epidemiological and Virological Characterization of Influenza B Virus Infections. PLoS ONE. 2016;11:e0161195. doi: 10.1371/journal.pone.0161195. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

