Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson's disease
- PMID: 33557896
- PMCID: PMC7869249
- DOI: 10.1186/s13024-021-00427-6
Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson's disease
Abstract
Background: Previous studies have reported that gut microbiota, permeability, short-chain fatty acids (SCFAs), and inflammation are altered in Parkinson's disease (PD), but how these factors are linked and how they contribute to disease processes and symptoms remains uncertain. This study sought to compare and identify associations among these factors in PD patients and controls to elucidate their interrelations and links to clinical manifestations of PD.
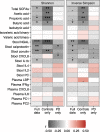
Methods: Stool and plasma samples and clinical data were collected from 55 PD patients and 56 controls. Levels of stool SCFAs and stool and plasma inflammatory and permeability markers were compared between patients and controls and related to one another and to the gut microbiota.
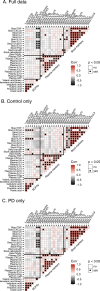
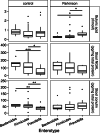
Results: Calprotectin was increased and SCFAs decreased in stool in PD in a sex-dependent manner. Inflammatory markers in plasma and stool were neither intercorrelated nor strongly associated with SCFA levels. Age at PD onset was positively correlated with SCFAs and negatively correlated with CXCL8 and IL-1β in stool. Fecal zonulin correlated positively with fecal NGAL and negatively with PD motor and non-motor symptoms. Microbiota diversity and composition were linked to levels of SCFAs, inflammatory factors, and zonulin in stool. Certain relationships differed between patients and controls and by sex.
Conclusions: Intestinal inflammatory responses and reductions in fecal SCFAs occur in PD, are related to the microbiota and to disease onset, and are not reflected in plasma inflammatory profiles. Some of these relationships are distinct in PD and are sex-dependent. This study revealed potential alterations in microbiota-host interactions and links between earlier PD onset and intestinal inflammatory responses and reduced SCFA levels, highlighting candidate molecules and pathways which may contribute to PD pathogenesis and clinical presentation and which warrant further investigation.
Keywords: Inflammation; Intestine; Microbiota; Parkinson’s disease; Short-chain fatty acids.
Conflict of interest statement
VTEA, PABP, LP, PA, and FS have patents issued (FI127671B & US10139408B2) and pending (US16/186,663 & EP3149205) that are assigned to NeuroBiome Ltd.
FS is founder and CEO of NeuroInnovation Oy and NeuroBiome Ltd., is a member of the scientific advisory board and has received consulting fees and stock options from Axial Biotherapeutics.
MGT is an advisor to INmune Bio, Longevity Biotech, Prevail Therapeutics, and Weston Garfield Foundation. MGT has patents issued (US Pat. Nos. 7144987B1 and 7244823B2) and pending (US20150239951, WO2019067789, 62/901698, see efiling Ack37193677, 62/905747, see efilingAck37274773) for co-invention of DN-TNFs.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

