Detecting Alzheimer's disease biomarkers with a brief tablet-based cognitive battery: sensitivity to Aβ and tau PET
- PMID: 33557905
- PMCID: PMC7871372
- DOI: 10.1186/s13195-021-00776-w
Detecting Alzheimer's disease biomarkers with a brief tablet-based cognitive battery: sensitivity to Aβ and tau PET
Abstract
Background: β-amyloid (Aβ) and tau positron emission tomography (PET) detect the pathological changes that define Alzheimer's disease (AD) in living people. Cognitive measures sensitive to Aβ and tau burden may help streamline identification of cases for confirmatory AD biomarker testing.
Methods: We examined the association of Brain Health Assessment (BHA) tablet-based cognitive measures with dichotomized Aβ -PET status using logistic regression models in individuals with mild cognitive impairment (MCI) or dementia (N = 140; 43 Aβ-, 97 Aβ+). We also investigated the relationship between the BHA tests and regional patterns of tau-PET signal using voxel-wise regression analyses in a subsample of 60 Aβ+ individuals with MCI or dementia.
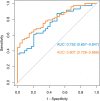
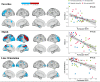
Results: Favorites (associative memory), Match (executive functions and speed), and Everyday Cognition Scale scores were significantly associated with Aβ positivity (area under the curve [AUC] = 0.75 [95% CI 0.66-0.85]). We found significant associations with tau-PET signal in mesial temporal regions for Favorites, frontoparietal regions for Match, and occipitoparietal regions for Line Orientation (visuospatial skills) in a subsample of individuals with MCI and dementia.
Conclusion: The BHA measures are significantly associated with both Aβ and regional tau in vivo imaging markers and could be used for the identification of patients with suspected AD pathology in clinical practice.
Keywords: Alzheimer’s disease; Biomarkers; Mild cognitive impairment; Neuropsychology; Positron emission tomography; Psychometrics.
Conflict of interest statement
None reported.
Figures

References
-
- U.S. Food and Drug Administration . Highlights of prescribing information: Amyvid (florbetapir F18 injection) Silver Spring, MD: Food and Drug Administration; 2012.
-
- U.S. Food and Drug Administration . FDA approves first drug to image tau pathology in patients being evaluated for Alzheimer’s disease. Silver Spring, MD: Food and Drug Administration; 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

