SARS-CoV-2 pneumonia-receptor binding and lung immunopathology: a narrative review
- PMID: 33557908
- PMCID: PMC7870126
- DOI: 10.1186/s13054-020-03399-z
SARS-CoV-2 pneumonia-receptor binding and lung immunopathology: a narrative review
Abstract
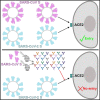
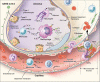
The current pandemic of COVID-19 caused thousands of deaths and healthcare professionals struggle to properly manage infected patients. This review summarizes information about SARS-CoV-2 receptor binding dynamics and intricacies, lung autopsy findings, immune response patterns, evidence-based explanations for the immune response, and COVID-19-associated hypercoagulability.
Keywords: ACE2; Acute respiratory distress syndrome; COVID-19; Hypercoagulability; Immunology; Pathology; SARS-CoV-2 pneumonia.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

