Cerebrospinal fluid N-224 tau helps discriminate Alzheimer's disease from subjective cognitive decline and other dementias
- PMID: 33557920
- PMCID: PMC7871566
- DOI: 10.1186/s13195-020-00756-6
Cerebrospinal fluid N-224 tau helps discriminate Alzheimer's disease from subjective cognitive decline and other dementias
Abstract
Background: Elevated cerebrospinal fluid (CSF) concentrations of total tau (T-tau) and phosphorylated tau at Thr181 (P-tau181) protein are typical of Alzheimer's disease (AD). However, the T-tau assay measures only the mid-region of the protein, while tau in CSF is instead composed of a series of fragments. One fragment species in particular, N-224, shows increased levels in AD compared to controls. In this multicentre study, we performed a clinical validation of the N-224 assay in cohorts including patients with subjective cognitive decline (SCD), mild cognitive impairment (MCI), AD, non-AD dementias and controls.
Methods: Cohorts consisted of 30 SCD and 30 probable AD from the Amsterdam Dementia Cohort (cohort 1) and 539 controls, 195 SCD, 232 MCI, 137 AD and 253 non-AD from the Swedish BioFINDER study (cohort 2). All samples had AD core biomarkers (Aβ42, T-tau, P-tau181) measurements. N-224 was measured with an in-house ultrasensitive Simoa assay.
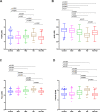
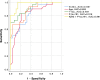
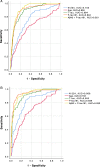
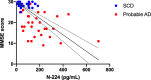
Results: N-224 levels were significantly higher in AD compared to SCD (cohort 1: p = 0.003) and in AD compared to all other diagnostic groups in cohort 2 (control, SCD, MCI and non-AD, p < 0.0001). Within the non-AD group, N-224 showed significantly lower concentrations compared to AD in Parkinson's disease (PD, p < 0.0001), Parkinson's disease dementia (PDD, p = 0.004), progressive supranuclear palsy (PSP, < 0.0001), multiple system atrophy (MSA, p = 0.002) and parkinsonisms not otherwise specified (NOS, p = 0.007). In cohort 1, higher concentrations of N-224 were associated to lower Mini-Mental State Examination (MMSE) scores (R2 = 0.318, β = 0.564, p ≤ 0.0001) and could accurately identify a pathological (< 24) MMSE score (p < 0.0001, AUC = 0.824).
Conclusions: N-224 tau can distinguish AD subjects from SCD and can discriminate subgroups of non-AD dementias from AD. Therefore, N-224 may be a useful addition to the tau biomarker toolbox for the study of tau species in CSF and for better understanding disease pathogenesis.
Keywords: Alzheimer’s disease; Biomarkers; Parkinsonisms; Subjective cognitive decline; Tau.
Conflict of interest statement
OH has acquired research support (for the institution) from AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, GE Healthcare, Pfizer and Roche. In the past 2 years, he has received consultancy/speaker fees from AC Immune, Alzpath, Biogen, Cerveau and Roche.PS has received consultancy/speaker fees (paid to the institution) from Biogen, Novartis Cardiology, Genentech and AC Immune. He is PI of studies with Vivoryon, EIP Pharma, IONIS, CogRx, AC Immune and FUJI-film/Toyama. HZ has served at scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics and CogRx; has given lectures in symposia sponsored by Fujirebio, Alzecure and Biogen; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). CET has a collaboration contract with ADx Neurosciences and performed contract research or received grants from Probiodrug, AC Immune, Biogen-Esai, CogRx, Toyama, Janssen prevention center, Boehringer, AxonNeurosciences, Fujirebio, EIP farma, PeopleBio and Roche. KB has served as a consultant or at advisory boards for Abcam, Axon, Biogen, Lilly, MagQu, Novartis and Roche Diagnostics and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work).
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

