Mechanism of miR-218-5p in autophagy, apoptosis and oxidative stress in rheumatoid arthritis synovial fibroblasts is mediated by KLF9 and JAK/STAT3 pathways
- PMID: 33558275
- PMCID: PMC8020083
- DOI: 10.1136/jim-2020-001437
Mechanism of miR-218-5p in autophagy, apoptosis and oxidative stress in rheumatoid arthritis synovial fibroblasts is mediated by KLF9 and JAK/STAT3 pathways
Abstract
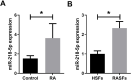
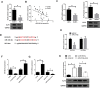
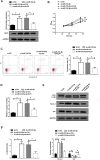
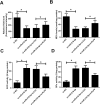
This study was aimed to investigate the effects of miR-218-5p on the proliferation, apoptosis, autophagy, and oxidative stress of rheumatoid arthritis synovial fibroblasts (RASFs), and the related mechanisms. Quantitative reverse transcription-PCR showed that the expression of miR-218-5p in rheumatoid arthritis synovial tissue was significantly higher than that in healthy synovial tissue. Compared with healthy synovial fibroblasts, miR-218-5p expression was obviously upregulated in RASFs, while KLF9 protein expression was markedly downregulated. Mechanistically, miR-218-5p could directly bind to the 3' untranslated region of KLF9 to inhibit the expression of KLF9. Additionally, transfection of miR-218-5p small interfering RNA (siRNA) inhibited the proliferation but promoted apoptosis and autophagy of RASFs. Simultaneously, miR-218-5p silencing reduced reactive oxygen species and malondialdehyde levels and increased superoxide dismutase and glutathione peroxidase activity to improve oxidative stress in RASFs. More importantly, the introduction of KLF9 siRNA reversed the effects of miR-218-5p siRNA transfection on RASF proliferation, apoptosis, autophagy, and oxidative stress. What is more, silencing miR-218-5p inhibited the activation of JAK2/STAT3 signaling pathway by targeting KLF9. Collectively, knockdown of miR-218-5p could regulate the proliferation, apoptosis, autophagy and oxidative stress of RASFs by increasing the expression of KLF9 and inhibiting the activation of the JAK2/STAT3 signaling pathway, which may provide a potential target for the mechanism research of RA.
Keywords: apoptosis.
© American Federation for Medical Research 2021. Re-use permitted under CC BY-NC. No commercial re-use. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
MiR-613 inhibits proliferation and invasion and induces apoptosis of rheumatoid arthritis synovial fibroblasts by direct down-regulation of DKK1.Cell Mol Biol Lett. 2019 Apr 19;24:8. doi: 10.1186/s11658-018-0130-0. eCollection 2019. Cell Mol Biol Lett. 2019. PMID: 31019537 Free PMC article.
-
MiR-140-5p inhibits synovial fibroblasts proliferation and inflammatory cytokines secretion through targeting TLR4.Biomed Pharmacother. 2017 Dec;96:208-214. doi: 10.1016/j.biopha.2017.09.079. Epub 2017 Oct 6. Biomed Pharmacother. 2017. PMID: 28987944
-
MicroRNA-449 targets histone deacetylase 1 to regulate the proliferation, invasion, and apoptosis of synovial fibroblasts in rheumatoid arthritis.Ann Palliat Med. 2021 Jul;10(7):7960-7969. doi: 10.21037/apm-21-1383. Ann Palliat Med. 2021. PMID: 34353082
-
MicroRNA-126 affects rheumatoid arthritis synovial fibroblast proliferation and apoptosis by targeting PIK3R2 and regulating PI3K-AKT signal pathway.Oncotarget. 2016 Nov 8;7(45):74217-74226. doi: 10.18632/oncotarget.12487. Oncotarget. 2016. PMID: 27729613 Free PMC article.
-
MiR-338-5p suppresses rheumatoid arthritis synovial fibroblast proliferation and invasion by targeting ADAMTS-9.Clin Exp Rheumatol. 2018 Mar-Apr;36(2):195-202. Epub 2017 Aug 28. Clin Exp Rheumatol. 2018. PMID: 28850027
Cited by
-
Hsa_circ_0000069 Accelerates Cervical Cancer Progression by Sponging miR-1270 to Facilitate CPEB4 Expression.Biochem Genet. 2024 Jun;62(3):1638-1656. doi: 10.1007/s10528-023-10494-7. Epub 2023 Sep 4. Biochem Genet. 2024. PMID: 37667097
-
MiR-218-5p promotes trophoblast infiltration and inhibits endoplasmic reticulum/oxidative stress by reducing UBE3A-mediated degradation of SATB1.J Cell Commun Signal. 2023 Sep;17(3):993-1008. doi: 10.1007/s12079-023-00751-0. Epub 2023 May 16. J Cell Commun Signal. 2023. PMID: 37191839 Free PMC article.
-
Sparse Independence Component Analysis for Competitive Endogenous RNA Co-Module Identification in Liver Hepatocellular Carcinoma.IEEE J Transl Eng Health Med. 2023 Jun 7;11:384-393. doi: 10.1109/JTEHM.2023.3283519. eCollection 2023. IEEE J Transl Eng Health Med. 2023. PMID: 37465460 Free PMC article.
-
Multiomics analysis of rheumatoid arthritis yields sequence variants that have large effects on risk of the seropositive subset.Ann Rheum Dis. 2022 Aug;81(8):1085-1095. doi: 10.1136/annrheumdis-2021-221754. Epub 2022 Apr 25. Ann Rheum Dis. 2022. PMID: 35470158 Free PMC article.
-
Identification of Tissue-Specific Expressed Hub Genes and Potential Drugs in Rheumatoid Arthritis Using Bioinformatics Analysis.Front Genet. 2022 Mar 18;13:855557. doi: 10.3389/fgene.2022.855557. eCollection 2022. Front Genet. 2022. PMID: 35368701 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

