Mitochondria: Powering the Innate Immune Response to Mycobacterium tuberculosis Infection
- PMID: 33558322
- PMCID: PMC8090963
- DOI: 10.1128/IAI.00687-20
Mitochondria: Powering the Innate Immune Response to Mycobacterium tuberculosis Infection
Abstract
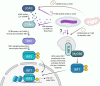
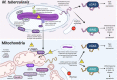
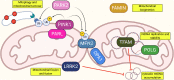
Within the last decade, we have learned that damaged mitochondria activate many of the same innate immune pathways that evolved to sense and respond to intracellular pathogens. These shared responses include cytosolic nucleic acid sensing and type I interferon (IFN) expression, inflammasome activation that leads to pyroptosis, and selective autophagy (called mitophagy when mitochondria are the cargo). Because mitochondria were once bacteria, parallels between how cells respond to mitochondrial and bacterial ligands are not altogether surprising. However, the potential for cross talk or synergy between bacterium- and mitochondrion-driven innate immune responses during infection remains poorly understood. This interplay is particularly striking, and intriguing, in the context of infection with the intracellular bacterial pathogen Mycobacterium tuberculosis (Mtb). Multiple studies point to a role for Mtb infection and/or specific Mtb virulence factors in disrupting the mitochondrial network in macrophages, leading to metabolic changes and triggering potent innate immune responses. Research from our laboratories and others argues that mutations in mitochondrial genes can exacerbate mycobacterial disease severity by hyperactivating innate responses or activating them at the wrong time. Indeed, growing evidence supports a model whereby different mitochondrial defects or mutations alter Mtb infection outcomes in distinct ways. By synthesizing the current literature in this minireview, we hope to gain insight into the molecular mechanisms driving, and consequences of, mitochondrion-dependent immune polarization so that we might better predict tuberculosis patient outcomes and develop host-directed therapeutics designed to correct these imbalances.
Keywords: LRRK2; Mycobacterium tuberculosis; cytosolic DNA sensing; innate immunity; macrophage; mtDNA; type I interferon.
Copyright © 2021 American Society for Microbiology.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

