Mammalian DNA Replication Timing
- PMID: 33558366
- PMCID: PMC8247564
- DOI: 10.1101/cshperspect.a040162
Mammalian DNA Replication Timing
Abstract
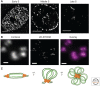
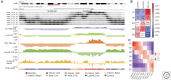
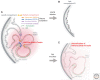
Immediately following the discovery of the structure of DNA and the semi-conservative replication of the parental DNA sequence into two new DNA strands, it became apparent that DNA replication is organized in a temporal and spatial fashion during the S phase of the cell cycle, correlated with the large-scale organization of chromatin in the nucleus. After many decades of limited progress, technological advances in genomics, genome engineering, and imaging have finally positioned the field to tackle mechanisms underpinning the temporal and spatial regulation of DNA replication and the causal relationships between DNA replication and other features of large-scale chromosome structure and function. In this review, we discuss these major recent discoveries as well as expectations for the coming decade.
Copyright © 2021 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
