AT-RvD1 Mitigates Secondhand Smoke-Exacerbated Pulmonary Inflammation and Restores Secondhand Smoke-Suppressed Antibacterial Immunity
- PMID: 33558371
- PMCID: PMC7952037
- DOI: 10.4049/jimmunol.2001228
AT-RvD1 Mitigates Secondhand Smoke-Exacerbated Pulmonary Inflammation and Restores Secondhand Smoke-Suppressed Antibacterial Immunity
Abstract
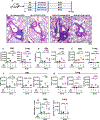
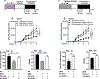
Cigarette smoke is a potent proinflammatory trigger contributing to acute lung injury and the development of chronic lung diseases via mechanisms that include the impairment of inflammation resolution. We have previously demonstrated that secondhand smoke (SHS) exposure exacerbates bacterial infection-induced pulmonary inflammation and suppresses immune responses. It is now recognized that resolution of inflammation is a bioactive process mediated by lipid-derived specialized proresolving mediators that counterregulate proinflammatory signaling and promote resolution pathways. We therefore hypothesized that proresolving mediators could reduce the burden of inflammation due to chronic lung infection following SHS exposure and restore normal immune responses to respiratory pathogens. To address this question, we exposed mice to SHS followed by chronic infection with nontypeable Haemophilus influenzae (NTHI). Some groups of mice were treated with aspirin-triggered resolvin D1 (AT-RvD1) during the latter half of the smoke exposure period or during a period of smoking cessation and before infection. Treatment with AT-RvD1 markedly reduced the recruitment of neutrophils, macrophages, and T cells in lung tissue and bronchoalveolar lavage and levels of proinflammatory cytokines in the bronchoalveolar lavage. Additionally, treatment with AT-RvD1 improved Ab titers against the NTHI outer membrane lipoprotein Ag P6 following infection. Furthermore, treatment with AT-RvD1 prior to classically adjuvanted immunization with P6 increased Ag-specific Ab titers, resulting in rapid clearance of NTHI from the lungs after acute challenge. Collectively, we have demonstrated that AT-RvD1 potently reverses the detrimental effects of SHS on pulmonary inflammation and immunity and thus could be beneficial in reducing lung injury associated with smoke exposure and infection.
Copyright © 2021 by The American Association of Immunologists, Inc.
Conflict of interest statement
Disclosures
The authors have no financial conflicts of interest.
Figures




References
-
- World Health Organization. 2016. Tobacco – fact sheet. Available at: http://www.who.int/mediacentre/factsheets/fs339/en/. Accessed: July 26, 2019.
-
- U.S. Department of Health and Human Services. 2014. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Atlanta, GA.
-
- Stampfli MR, and Anderson GP. 2009. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat. Rev. Immunol. 9: 377–384. - PubMed
-
- Oberg M, Jaakkola MS, Woodward A, Peruga A, and Pruss-Ustun A. 2011. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet. 377: 139–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

