Treatment Outcomes and Clinical Characteristics of Patients with KRAS-G12C-Mutant Non-Small Cell Lung Cancer
- PMID: 33558425
- PMCID: PMC8771577
- DOI: 10.1158/1078-0432.CCR-20-4023
Treatment Outcomes and Clinical Characteristics of Patients with KRAS-G12C-Mutant Non-Small Cell Lung Cancer
Abstract
Purpose: KRAS mutations are identified in approximately 30% of patients with non-small cell lung cancer (NSCLC). Novel direct inhibitors of KRAS G12C have shown activity in early-phase clinical trials. We hypothesized that patients with KRAS G12C mutations may have distinct clinical characteristics and responses to therapies.
Experimental design: Through routine next-generation sequencing, we identified patients with KRAS-mutant NSCLC treated at Memorial Sloan Kettering Cancer Center (New York, NY) from 2014 to 2018 and reviewed tumor characteristics, overall survival, and treatment outcomes.
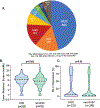
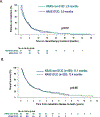
Results: We identified 1,194 patients with KRAS-mutant NSCLC, including 770 with recurrent or metastatic disease. KRAS G12C mutations were present in 46% and KRAS non-G12C mutations in 54%. Patients with KRAS G12C had a higher tumor mutation burden (median, 8.8 vs. 7 mut/Mb; P = 0.006) and higher median PD-L1 expression (5% vs. 1%). The comutation patterns of STK11 (28% vs. 29%) and KEAP1 (23% vs. 24%) were similar. The median overall survivals from diagnosis were similar for KRAS G12C (13.4 months) and KRAS non-G12C mutations (13.1 months; P = 0.96). In patients with PD-L1 ≥50%, there was not a significant difference in response rate with single-agent immune checkpoint inhibitor for patients with KRAS G12C mutations (40% vs. 58%; P = 0.07).
Conclusions: We provide outcome data for a large series of patients with KRAS G12C-mutant NSCLC with available therapies, demonstrating that responses and duration of benefit with available therapies are similar to those seen in patients with KRAS non-G12C mutations. Strategies to incorporate new targeted therapies into the current treatment paradigm will need to consider outcomes specific to patients harboring KRAS G12C mutations.
©2021 American Association for Cancer Research.
Figures

References
-
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N Engl J Med. 2016;375(19):1823–1833. - PubMed
-
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N Engl J Med. 2018;378(22):2078–2092. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

