Characterization of ferroptosis in kidney tubular cell death under diabetic conditions
- PMID: 33558472
- PMCID: PMC7870666
- DOI: 10.1038/s41419-021-03452-x
Characterization of ferroptosis in kidney tubular cell death under diabetic conditions
Erratum in
-
Correction: Characterization of ferroptosis in kidney tubular cell death under diabetic conditions.Cell Death Dis. 2021 Apr 8;12(4):382. doi: 10.1038/s41419-021-03667-y. Cell Death Dis. 2021. PMID: 33833214 Free PMC article. No abstract available.
Abstract
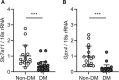
Kidney tubular cell death induced by transforming growth factor-β1 (TGF-β1) is known to contribute to diabetic nephropathy, a major complication of diabetes. Caspase-3-dependent apoptosis and caspase-1-dependent pyroptosis are also involved in tubular cell death under diabetic conditions. Recently, ferroptosis, an atypical form of iron-dependent cell death, was reported to cause kidney disease, including acute kidney injury. Ferroptosis is primed by lipid peroxide accumulation through the cystine/glutamate antiporter system Xc- (xCT) and glutathione peroxidase 4 (GPX4)-dependent mechanisms. The aim of this study was to evaluate the role of ferroptosis in diabetes-induced tubular injury. TGF-β1-stimulated proximal tubular epithelial cells and diabetic mice models were used for in vitro and in vivo experiments, respectively. xCT and GPX4 expression, cell viability, glutathione concentration, and lipid peroxidation were quantified to indicate ferroptosis. The effect of ferroptosis inhibition was also assessed. In kidney biopsy samples from diabetic patients, xCT and GPX4 mRNA expression was decreased compared to nondiabetic samples. In TGF-β1-stimulated tubular cells, intracellular glutathione concentration was reduced and lipid peroxidation was enhanced, both of which are related to ferroptosis-related cell death. Ferrostatin-1 (Fer-1), a ferroptosis inhibitor, alleviated TGF-β1-induced ferroptosis. In diabetic mice, kidney mRNA and protein expressions of xCT and GPX4 were reduced compared to control. Kidney glutathione concentration was decreased, while lipid peroxidation was increased in these mice, and these changes were alleviated by Fer-1 treatment. Ferroptosis is involved in kidney tubular cell death under diabetic conditions. Ferroptosis inhibition could be a therapeutic option for diabetic nephropathy.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

