Sequence-encoded and composition-dependent protein-RNA interactions control multiphasic condensate morphologies
- PMID: 33558506
- PMCID: PMC7870978
- DOI: 10.1038/s41467-021-21089-4
Sequence-encoded and composition-dependent protein-RNA interactions control multiphasic condensate morphologies
Abstract
Multivalent protein-protein and protein-RNA interactions are the drivers of biological phase separation. Biomolecular condensates typically contain a dense network of multiple proteins and RNAs, and their competing molecular interactions play key roles in regulating the condensate composition and structure. Employing a ternary system comprising of a prion-like polypeptide (PLP), arginine-rich polypeptide (RRP), and RNA, we show that competition between the PLP and RNA for a single shared partner, the RRP, leads to RNA-induced demixing of PLP-RRP condensates into stable coexisting phases-homotypic PLP condensates and heterotypic RRP-RNA condensates. The morphology of these biphasic condensates (non-engulfing/ partial engulfing/ complete engulfing) is determined by the RNA-to-RRP stoichiometry and the hierarchy of intermolecular interactions, providing a glimpse of the broad range of multiphasic patterns that are accessible to these condensates. Our findings provide a minimal set of physical rules that govern the composition and spatial organization of multicomponent and multiphasic biomolecular condensates.
Conflict of interest statement
The authors declare no competing interests.
Figures

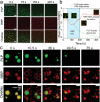



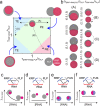
References
-
- Shin, Y. & Brangwynne, C. P. Liquid phase condensation in cell physiology and disease. Science10.1126/science.aaf4382 (2017). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

