Rapid decline of neutralizing antibodies against SARS-CoV-2 among infected healthcare workers
- PMID: 33558507
- PMCID: PMC7870823
- DOI: 10.1038/s41467-021-21111-9
Rapid decline of neutralizing antibodies against SARS-CoV-2 among infected healthcare workers
Erratum in
-
Author Correction: Rapid decline of neutralizing antibodies against SARS-CoV-2 among infected healthcare workers.Nat Commun. 2021 May 10;12(1):2824. doi: 10.1038/s41467-021-23128-6. Nat Commun. 2021. PMID: 33972561 Free PMC article. No abstract available.
Abstract
There are only few data concerning persistence of neutralizing antibodies (NAbs) among SARS-CoV-2-infected healthcare workers (HCW). These individuals are particularly exposed to SARS-CoV-2 infection and at potential risk of reinfection. We followed 26 HCW with mild COVID-19 three weeks (D21), two months (M2) and three months (M3) after the onset of symptoms. All the HCW had anti-receptor binding domain (RBD) IgA at D21, decreasing to 38.5% at M3 (p < 0.0001). Concomitantly a significant decrease in NAb titers was observed between D21 and M2 (p = 0.03) and between D21 and M3 (p < 0.0001). Here, we report that SARS-CoV-2 can elicit a NAb response correlated with anti-RBD antibody levels. However, this neutralizing activity declines, and may even be lost, in association with a decrease in systemic IgA antibody levels, from two months after disease onset. This short-lasting humoral protection supports strong recommendations to maintain infection prevention and control measures in HCW, and suggests that periodic boosts of SARS-CoV-2 vaccination may be required.
Conflict of interest statement
The authors declare no competing interests.
Figures
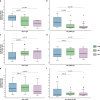
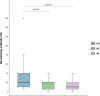

References
-
- Center for Disease Control and Prevention. Infection Control Guidance for Healthcare Professionals about Coronavirus (COVID-19) (2020).
-
- European Centre for Disease Prevention and Control. Infection Prevention and Control and Preparedness for COVID-19 in Healthcare Settings—Third Update. Technical Report (2020).
-
- World Health Organization. Rational use of personal protective equipment for coronavirus disease (COVID-19) and considerations during severe shortages. Interim guidance (2020).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

