The mechanosensitive Piezo1 channel mediates heart mechano-chemo transduction
- PMID: 33558521
- PMCID: PMC7870949
- DOI: 10.1038/s41467-021-21178-4
The mechanosensitive Piezo1 channel mediates heart mechano-chemo transduction
Abstract
The beating heart possesses the intrinsic ability to adapt cardiac output to changes in mechanical load. The century-old Frank-Starling law and Anrep effect have documented that stretching the heart during diastolic filling increases its contractile force. However, the molecular mechanotransduction mechanism and its impact on cardiac health and disease remain elusive. Here we show that the mechanically activated Piezo1 channel converts mechanical stretch of cardiomyocytes into Ca2+ and reactive oxygen species (ROS) signaling, which critically determines the mechanical activity of the heart. Either cardiac-specific knockout or overexpression of Piezo1 in mice results in defective Ca2+ and ROS signaling and the development of cardiomyopathy, demonstrating a homeostatic role of Piezo1. Piezo1 is pathologically upregulated in both mouse and human diseased hearts via an autonomic response of cardiomyocytes. Thus, Piezo1 serves as a key cardiac mechanotransducer for initiating mechano-chemo transduction and consequently maintaining normal heart function, and might represent a novel therapeutic target for treating human heart diseases.
Conflict of interest statement
The authors declare no competing interests.
Figures
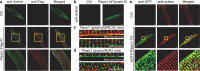
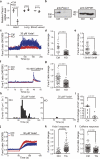

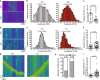
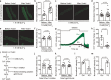



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

