Pentapartite fractionation of particles in oral fluids by differential centrifugation
- PMID: 33558596
- PMCID: PMC7870959
- DOI: 10.1038/s41598-021-82451-6
Pentapartite fractionation of particles in oral fluids by differential centrifugation
Abstract
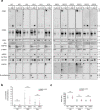
Oral fluids (OFs) contain small extracellular vesicles (sEVs or exosomes) that carry disease-associated diagnostic molecules. However, cells generate extracellular vesicles (EVs) other than sEVs, so the EV population is quite heterogeneous. Furthermore, molecules not packaged in EVs can also serve as diagnostic markers. For these reasons, developing a complete picture of particulate matter in the oral cavity is important before focusing on specific subtypes of EVs. Here, we used differential centrifugation to fractionate human OFs from healthy volunteers and patients with oral squamous cell carcinoma into 5 fractions, and we characterized the particles, nucleic acids, and proteins in each fraction. Canonical exosome markers, including CD63, CD9, CD133, and HSP70, were found in all fractions, whereas CD81 and AQP5 were enriched in the 160K fraction, with non-negligible amounts in the 2K fraction. The 2K fraction also contained its characteristic markers that included short derivatives of EGFR and E-cadherin, as well as an autophagosome marker, LC3, and large multi-layered vesicles were observed by electronic microscopy. Most of the DNA and RNA was recovered from the 0.3K and 2K fractions, with some in the 160K fraction. These results can provide guideline information for development of purpose-designed OF-based diagnostic systems.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

