Berberine alleviates visceral hypersensitivity in rats by altering gut microbiome and suppressing spinal microglial activation
- PMID: 33558654
- PMCID: PMC8563748
- DOI: 10.1038/s41401-020-00601-4
Berberine alleviates visceral hypersensitivity in rats by altering gut microbiome and suppressing spinal microglial activation
Abstract
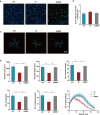
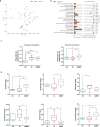
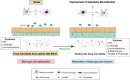
Accumulating evidence shows that agents targeting gut dysbiosis are effective for improving symptoms of irritable bowel syndrome (IBS). However, the potential mechanisms remain unclear. In this study we investigated the effects of berberine on the microbiota-gut-brain axis in two rat models of visceral hypersensitivity, i.e., specific pathogen-free SD rats subjected to chronic water avoidance stress (WAS) and treated with berberine (200 mg· kg-1 ·d-1, ig, for 10 days) as well as germ-free (GF) rats subjected to fecal microbiota transplantation (FMT) from a patient with IBS (designated IBS-FMT) and treated with berberine (200 mg· kg-1 ·d-1, ig, for 2 weeks). Before the rats were sacrificed, visceral sensation and depressive behaviors were evaluated. Then colonic tryptase was measured and microglial activation in the dorsal lumbar spinal cord was assessed. The fecal microbiota was profiled using 16S rRNA sequencing, and short chain fatty acids (SCFAs) were measured. We showed that berberine treatment significantly alleviated chronic WAS-induced visceral hypersensitivity and activation of colonic mast cells and microglia in the dorsal lumbar spinal cord. Transfer of fecal samples from berberine-treated stressed donors to GF rats protected against acute WAS. FMT from a patient with IBS induced visceral hypersensitivity and pro-inflammatory phenotype in microglia, while berberine treatment reversed the microglial activation and altered microbial composition and function and SCFA profiles in stools of IBS-FMT rats. We demonstrated that berberine did not directly influence LPS-induced microglial activation in vitro. In both models, several SCFA-producing genera were enriched by berberine treatment, and positively correlated to the morphological parameters of microglia. In conclusion, activation of microglia in the dorsal lumbar spinal cord was involved in the pathogenesis of IBS caused by dysregulation of the microbiota-gut-brain axis, and the berberine-altered gut microbiome mediated the modulatory effects of the agent on microglial activation and visceral hypersensitivity, providing a potential option for the treatment of IBS.
Keywords: berberine; gut microbiome; microbiota-gut-brain axis; microglia; spinal cord; visceral hypersensitivity.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




Similar articles
-
Mechanosensory Piezo2 regulated by gut microbiota participates in the development of visceral hypersensitivity and intestinal dysmotility.Gut Microbes. 2025 Dec;17(1):2497399. doi: 10.1080/19490976.2025.2497399. Epub 2025 Apr 28. Gut Microbes. 2025. PMID: 40296251 Free PMC article.
-
Rifaximin-mediated gut microbiota regulation modulates the function of microglia and protects against CUMS-induced depression-like behaviors in adolescent rat.J Neuroinflammation. 2021 Nov 4;18(1):254. doi: 10.1186/s12974-021-02303-y. J Neuroinflammation. 2021. PMID: 34736493 Free PMC article.
-
Effect of water extracts from Cynanchum thesioides (Freyn) K. Schum. on visceral hypersensitivity and gut microbiota profile in maternally separated rats.J Ethnopharmacol. 2021 Jan 10;264:113352. doi: 10.1016/j.jep.2020.113352. Epub 2020 Sep 3. J Ethnopharmacol. 2021. PMID: 32891821
-
Stress and the Microbiota-Gut-Brain Axis in Visceral Pain: Relevance to Irritable Bowel Syndrome.CNS Neurosci Ther. 2016 Feb;22(2):102-17. doi: 10.1111/cns.12490. Epub 2015 Dec 10. CNS Neurosci Ther. 2016. PMID: 26662472 Free PMC article. Review.
-
Irritable bowel syndrome, the gut microbiome, and diet.Curr Opin Endocrinol Diabetes Obes. 2025 Jun 1;32(3):102-107. doi: 10.1097/MED.0000000000000905. Epub 2025 Feb 19. Curr Opin Endocrinol Diabetes Obes. 2025. PMID: 39968682 Review.
Cited by
-
N,N,N',N'-Tetrakis(2-pyridylmethyl)ethylenediamine induces endothelium-dependent hyperpolarization-mediated vasorelaxation via store-operated calcium entry mechanism in healthy and intestinal inflammatory mice.Heliyon. 2024 Jul 6;10(14):e33994. doi: 10.1016/j.heliyon.2024.e33994. eCollection 2024 Jul 30. Heliyon. 2024. PMID: 39108891 Free PMC article.
-
The Gut Microbiota and Chronic Pain.Curr Pain Headache Rep. 2024 Apr;28(4):259-269. doi: 10.1007/s11916-024-01221-x. Epub 2024 Feb 12. Curr Pain Headache Rep. 2024. PMID: 38345694 Review.
-
The gut-masticatory muscles-temporomandibular joint pain axis-a scoping review.J Oral Facial Pain Headache. 2025 Mar;39(1):24-33. doi: 10.22514/jofph.2025.021. Epub 2025 Mar 12. J Oral Facial Pain Headache. 2025. PMID: 40129421 Free PMC article.
-
The Potential Prebiotic Berberine Combined With Methimazole Improved the Therapeutic Effect of Graves' Disease Patients Through Regulating the Intestinal Microbiome.Front Immunol. 2022 Jan 10;12:826067. doi: 10.3389/fimmu.2021.826067. eCollection 2021. Front Immunol. 2022. PMID: 35082799 Free PMC article.
-
Evaluation of two laboratory model methods for diarrheal irritable bowel syndrome.Mol Med. 2023 Jan 12;29(1):5. doi: 10.1186/s10020-022-00599-x. Mol Med. 2023. PMID: 36635623 Free PMC article.
References
-
- Drossman DA, Hasler WL. Rome IV-functional GI disorders: disorders of gut-brain interaction. Gastroenterology. 2016;150:1257–61.. - PubMed
-
- Ford AC, Lacy BE, Talley NJ. Irritable bowel syndrome. N Engl J Med. 2017;376:2566–78.. - PubMed
-
- Koloski NA, Jones M, Talley NJ. Evidence that independent gut-to-brain and brain-to-gut pathways operate in the irritable bowel syndrome and functional dyspepsia: a 1-year population-based prospective study. Aliment Pharmacol Ther. 2016;44:592–600. - PubMed
-
- Wilcz-Villega EM, McClean S, O’Sullivan MA. Mast cell tryptase reduces junctional adhesion molecule-A (JAM-A) expression in intestinal epithelial cells: implications for the mechanisms of barrier dysfunction in irritable bowel syndrome. Am J Gastroenterol. 2013;108:1140–51.. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

