Treatment of nonmetastatic castration-resistant prostate cancer: focus on second-generation androgen receptor inhibitors
- PMID: 33558665
- PMCID: PMC8134049
- DOI: 10.1038/s41391-020-00310-3
Treatment of nonmetastatic castration-resistant prostate cancer: focus on second-generation androgen receptor inhibitors
Abstract
Background: Nonmetastatic castration-resistant prostate cancer (nmCRPC) is defined as a rising prostate-specific antigen concentration, despite castrate levels of testosterone with ongoing androgen-deprivation therapy or orchiectomy, and no detectable metastases by conventional imaging. Patients with nmCRPC progress to metastatic disease and are at risk of developing cancer-related symptoms and morbidity, eventually dying of their disease. While patients with nmCRPC are generally asymptomatic from their disease, they are often older and have chronic comorbidities that require long-term concomitant medication. Therefore, careful consideration of the benefit-risk profile of potential treatments is required.
Methods: In this review, we will discuss the rationale for early treatment of patients with nmCRPC to delay metastatic progression and prolong survival, as well as the factors influencing this treatment decision. We will focus on oral pharmacotherapy with the second-generation androgen receptor inhibitors, apalutamide, enzalutamide, and darolutamide, and the importance of balancing the clinical benefit they offer with potential adverse events and the consequential impact on quality of life, physical capacity, and cognitive function.
Results and conclusions: While the definition of nmCRPC is well established, the advent of next-generation imaging techniques capable of detecting hitherto undetectable oligometastatic disease in patients with nmCRPC has fostered debate on the criteria that inform the management of these patients. However, despite these developments, published consensus statements have maintained that the absence of metastases on conventional imaging suffices to guide such therapeutic decisions. In addition, the prolonged metastasis-free survival and recently reported positive overall survival outcomes of the three second-generation androgen receptor inhibitors have provided further evidence for the early use of these agents in patients with nmCRPC in order to delay metastases and prolong survival. Here, we discuss the benefit-risk profiles of apalutamide, enzalutamide, and darolutamide based on the data available from their pivotal clinical trials in patients with nmCRPC.
Conflict of interest statement
FS has received honoraria and has served as an advisor for Bayer, Astellas, Janssen, Amgen, AstraZeneca, and Sanofi, and his institution has received research grants from Bayer, Astellas, Janssen, Amgen, AstraZeneca, BMS, and Sanofi. MB has received honoraria and has served as an advisor for Astellas, Bayer, Janssen, Amgen, AstraZeneca, Lilly, MSD, Pfizer, EUSApharm, Eisai, Ipsen, BMS, Merck, Novartis, and Sanofi, and his institution has received research grants from Astellas and Janssen. KS has received honoraria and has served as an advisor for Bayer, Takeda, Astellas, Janssen, AstraZeneca, and Sanofi, and has received research grants from Takeda, Astellas, Sanofi, and Daiichi-Sankyo. NS has received honoraria and has served as an advisor for Amgen, Astellas, AstraZeneca, Bayer, BMS, Dendreon, Janssen, Merck, Pfizer, and Sanofi-Genzyme.
Figures

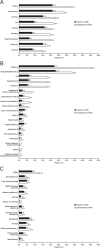

References
-
- Luo J, Beer TM, Graff JN. Treatment of nonmetastatic castration-resistant prostate cancer. Oncology. 2016;30:336–44. - PubMed
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Uchio EM, Aslan M, Wells CK, Calderone J, Concato J. Impact of biochemical recurrence in prostate cancer among US veterans. Arch Intern Med. 2010;170:1390–5. - PubMed
-
- National Comprehensive Cancer Network. National Comprehensive Cancer Network clinical practice guidelines in oncology: prostate cancer. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed 7 May 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

