Human heart-forming organoids recapitulate early heart and foregut development
- PMID: 33558697
- PMCID: PMC8192303
- DOI: 10.1038/s41587-021-00815-9
Human heart-forming organoids recapitulate early heart and foregut development
Erratum in
-
Publisher Correction: Human heart-forming organoids recapitulate early heart and foregut development.Nat Biotechnol. 2021 Jun;39(6):775. doi: 10.1038/s41587-021-00960-1. Nat Biotechnol. 2021. PMID: 34017142 Free PMC article. No abstract available.
Abstract
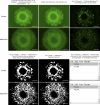
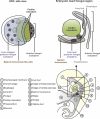
Organoid models of early tissue development have been produced for the intestine, brain, kidney and other organs, but similar approaches for the heart have been lacking. Here we generate complex, highly structured, three-dimensional heart-forming organoids (HFOs) by embedding human pluripotent stem cell aggregates in Matrigel followed by directed cardiac differentiation via biphasic WNT pathway modulation with small molecules. HFOs are composed of a myocardial layer lined by endocardial-like cells and surrounded by septum-transversum-like anlagen; they further contain spatially and molecularly distinct anterior versus posterior foregut endoderm tissues and a vascular network. The architecture of HFOs closely resembles aspects of early native heart anlagen before heart tube formation, which is known to require an interplay with foregut endoderm development. We apply HFOs to study genetic defects in vitro by demonstrating that NKX2.5-knockout HFOs show a phenotype reminiscent of cardiac malformations previously observed in transgenic mice.
Conflict of interest statement
The authors declare no competing interests.
Figures
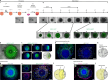



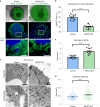


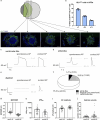


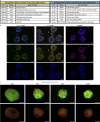

Comment in
-
Recapitulating early cardiogenesis in vitro.Nat Methods. 2021 Apr;18(4):331. doi: 10.1038/s41592-021-01118-2. Nat Methods. 2021. PMID: 33828266 No abstract available.
References
-
- Fatehullah, A., Tan, S. H. & Barker, N. Organoids as an in vitro model of human development and disease. Nat. Cell Biol.18, 246–254 (2016). - PubMed
-
- Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature459, 262–265 (2009). - PubMed
-
- Hansen, A. et al. Development of a drug screening platform based on engineered heart tissue. Circ. Res.107, 35–44 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

