Prolonged activation of innate immune pathways by a polyvalent STING agonist
- PMID: 33558734
- PMCID: PMC8126516
- DOI: 10.1038/s41551-020-00675-9
Prolonged activation of innate immune pathways by a polyvalent STING agonist
Erratum in
-
Author Correction: Prolonged activation of innate immune pathways by a polyvalent STING agonist.Nat Biomed Eng. 2021 May;5(5):483. doi: 10.1038/s41551-021-00741-w. Nat Biomed Eng. 2021. PMID: 33963308 Free PMC article. No abstract available.
Abstract
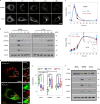
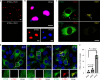
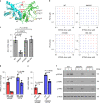
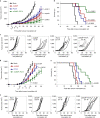
The stimulator of interferon genes (STING) is an endoplasmic reticulum transmembrane protein that is a target of therapeutics for infectious diseases and cancer. However, early-phase clinical trials of small-molecule STING agonists have shown limited antitumour efficacy and dose-limiting toxicity. Here, we show that a polyvalent STING agonist-a pH-sensitive polymer bearing a seven-membered ring with a tertiary amine (PC7A)-activates innate-immunity pathways through the polymer-induced formation of STING-PC7A condensates. In contrast to the natural STING ligand 2',3'-cyclic-GMP-AMP (cGAMP), PC7A stimulates the prolonged production of pro-inflammatory cytokines by binding to a non-competitive STING surface site that is distinct from the cGAMP binding pocket. PC7A induces antitumour responses that are dependent on STING expression and CD8+ T-cell activity, and the combination of PC7A and cGAMP led to synergistic therapeutic outcomes (including the activation of cGAMP-resistant STING variants) in mice bearing subcutaneous tumours and in resected human tumours and lymph nodes. The activation of the STING pathway through polymer-induced STING condensation may offer new therapeutic opportunities.
Conflict of interest statement
B.D.S. and J.G. are scientific co-founders and advisors of OncoNano Medicine, Inc.
Figures

References
-
- Yum S, Li M, Frankel AE, Chen ZJ. Roles of the cGAS-STING pathway in cancer immunosurveillance and immunotherapy. Annu. Rev. Cancer Biol. 2019;3:323–344. doi: 10.1146/annurev-cancerbio-030518-055636. - DOI
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

