Impact of serological and PCR testing requirements on the selection of COVID-19 convalescent plasma donors
- PMID: 33559248
- PMCID: PMC8013201
- DOI: 10.1111/trf.16293
Impact of serological and PCR testing requirements on the selection of COVID-19 convalescent plasma donors
Abstract
Background: Convalescent plasma is undergoing randomized trials as a potential therapeutic option for COVID-19 infection. Little empirical evidence exists regarding the determination of donor eligibility and experiences with donor selection.
Study design and methods: This prospective study was conducted at a tertiary care hospital in New York to select plasma donors for a randomized, double-blind, controlled convalescent plasma trial. Clearance for donation required successful completion of an online questionnaire and an in-person screening visit, which included (a) completion of a Donor Health Questionnaire (DHQ), (b) Immunoglobulin G (IgG) antibody testing using an immunochromatographic anti- severe acute respiratory coronavirus 2 (SARS-CoV-2) test, (c) Polymerase chain reaction (PCR) testing if <28 days from symptom resolution, and (d) routine blood bank testing.
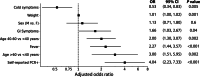
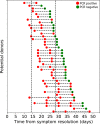
Results: After receiving 3093 online questionnaires, 521 individuals presented for in-person screening visits, with 40.1% (n = 209) fully qualifying. Subjects (n = 312) failed to progress due to the following reasons: disqualifying answer from DHQ (n = 30, 9.6%), insufficient antibodies (n = 198, 63.5%), persistent positive PCR tests (n = 14, 4.5%), and blood donation testing labs (n = 70, 22.4%). Importantly, 24.6% and 11.1% of potential donors who reported having PCR-diagnosed infection had low or undetectable SARS-CoV-2 antibody levels, respectively. Surprisingly, 62.9% (56/89) of subjects had positive PCR tests 14-27 days after symptom resolution, with 13 individuals continuing to be PCR positive after 27 days.
Conclusion: It is feasible for a single site to fully qualify a large number of convalescent plasma donors in a short period of time. Among otherwise qualified convalescent plasma donors, we found high rates of low or undetectable antibody levels and many individuals with persistently positive PCR tests.
Keywords: blood component preparations; donors; immunology (other than RBC serology).
© 2021 AABB.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures


References
-
- Mair‐Jenkins J, Saavedra‐Campos M, Baillie JK, Cleary P, Khaw F‐M, Lim WS, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta‐analysis. J Infect Dis. 2015;211:80–90. 10.1093/infdis/jiu396. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

