Predictors, Type, and Impact of Bleeding on the Net Clinical Benefit of Long-Term Ticagrelor in Stable Patients With Prior Myocardial Infarction
- PMID: 33559485
- PMCID: PMC7955333
- DOI: 10.1161/JAHA.120.017008
Predictors, Type, and Impact of Bleeding on the Net Clinical Benefit of Long-Term Ticagrelor in Stable Patients With Prior Myocardial Infarction
Abstract
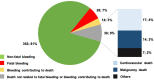
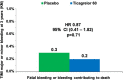
Background Ticagrelor reduces ischemic risk but increases bleeding in patients with prior myocardial infarction. Identification of patients at lower bleeding risk is important in selecting patients who are likely to derive more favorable outcomes versus risk from this strategy. Methods and Results PEGASUS-TIMI 54 (Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin-Thrombolysis in Myocardial Infarction 54) randomized 21 162 patients with prior myocardial infarction in a 1:1:1 fashion to ticagrelor 60 mg or 90 mg twice daily or placebo, with ticagrelor 60 mg approved for long-term use. TIMI major or minor bleeding was the primary end point for this analysis. Causes of bleeding were categorized by site and etiology, and independent predictors were identified. At 3 years, ticagrelor 60 mg increased the rate of TIMI major or minor bleeding by 2.0% versus placebo (1.4% placebo versus 3.4% ticagrelor). The bleeding excess was driven primarily by spontaneous gastrointestinal bleeds. A history of spontaneous bleeding requiring hospitalization and the presence of anemia were independent predictors of bleeding but not of ischemic risk. Patients with at least 1 risk predictor had 3-fold higher rates of bleeding with ticagrelor 60 mg versus those who had neither (absolute risk increase, 4.4% versus 1.5%; P=0.01). Patients with neither predictor had a more favorable benefit profile with ticagrelor 60 mg versus placebo including lower mortality (hazard ratio, 0.79; 95% CI, 0.65-0.96; P interaction = 0.03). Conclusions In patients with prior myocardial infarction, bleeding with ticagrelor 60 mg twice daily is predominantly spontaneous gastrointestinal. A history of spontaneous bleeding requiring hospitalization or the presence of anemia identifies patients at higher risk of bleeding, and the absence of either identifies patients likely to have a more favorable net benefit with ticagrelor. Registration URL https://www.clinicaltrials.gov/. Unique identifier: NCT01225562.
Keywords: benefit‐risk ratio; bleeding; long‐term ticagrelor; myocardial infarction.
Conflict of interest statement
The TIMI Study Group has received significant research grant support from Abbott, Amgen, Aralez, AstraZeneca, Bayer HealthCare Pharmaceuticals, Inc., BRAHMS, Daiichi‐Sankyo, Eisai, GlaxoSmithKline, Intarcia, Janssen, MedImmune, Merck, Novartis, Pfizer, Poxel, Quark Pharmaceuticals, Roche, Takeda, The Medicines Company, and Zora Biosciences. Giulia Magnani reports speaking fees from AstraZeneca, Daiichi Sankyo, consultancy fee from Boehringer Ingelheim. KyungAh Im is a member of the TIMI Study Group, which has received institutional research grant support through Brigham and Women’s Hospital from Abbott, Amgen, Aralez, AstraZeneca, Bayer HealthCare Pharmaceuticals, Inc., BRAHMS, Daiichi‐Sankyo, Eisai, GlaxoSmithKline, Intarcia, Janssen, MedImmune, Merck, Novartis, Pfizer, Poxel, Quark Pharmaceuticals, Roche, Takeda, The Medicines Company, and Zora Biosciences. Andrej Budaj reports personal fees and non‐financial support from AstraZeneca, during the conduct of the study; personal fees and non‐financial support from Bristol Myers Squibb/Pfizer, personal fees and non‐financial support from Bayer, personal fees and nonfinancial support from Sanofi Aventis, personal fees from Eisai, personal fees from Novartis, and personal fees from GlaxoSmithKline, outside the submitted work. Robert F Storey reports research grants, consultancy fees and honoraria from AstraZeneca; research grants and consultancy fees from PlaqueTec; consultancy fees and honoraria from Bayer; consultancy fees and honoraria from Bristol Myers Squibb/Pfizer alliance; and consultancy fees from Avacta, Idorsia, Haemonetics, Novartis, and Thromboserin. P. Gabriel Steg reports research grants from Amarin, Bayer, Merck, Sanofi, and Servier; and speaking or consulting fees from Amarin, Amgen, AstraZeneca, Bayer/Janssen, Boehringer‐Ingelheim, Bristol‐Myers‐Squibb, Idorsia, Lilly, Merck, Novartis, Novo‐Nordisk, Pfizer, Regeneron, Sanofi, and Servier. Deepak L. Bhatt reports the following relationships: Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, PhaseBio, and Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the [Portico Re‐sheathable Transcatheter Aortic Valve System US IDE Trial, PORTICO] trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the [Efficacy of Secukinumab Compared to Adalimumab in Patients with Psoriatic Arthritis, ExCEED] trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the [Edoxaban versus standard of care and their effects on clinical outcomes in patients having undergone transcatheter aortic valve implantation, ENVISAGE] trial, funded by Daiichi Sankyo), Population Health Research Institute; honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice‐Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; [Randomized Evaluation of Dual Antithrombotic Therapy with Dabigatran versus Triple Therapy with Warfarin in Patients with Nonvalvular Atrial Fibrillation Undergoing Percutaneous Coronary Intervention, RE‐DUAL] PCI clinical trial steering committee funded by Boehringer Ingelheim), Belvoir Publications (editor‐in‐chief,
Figures




Comment in
-
Long-Term Ticagrelor in Stable Patients With Prior Myocardial Infarction: Bleeding Avoidance First and Foremost.J Am Heart Assoc. 2021 Feb 16;10(4):e019889. doi: 10.1161/JAHA.120.019889. Epub 2021 Feb 9. J Am Heart Assoc. 2021. PMID: 33559475 Free PMC article. No abstract available.
References
-
- Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, Magnani G, Bansilal S, Fish MP, Im K, et al. PEGASUS‐TIMI 54 Steering Committee and Investigators. Long‐term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791–1800. - PubMed
-
- Dellborg M, Bonaca MP, Storey RF, Steg PG, Im KA, Cohen M, Bhatt DL, Oude Ophuis T, Budaj A, Hamm C, et al. Efficacy and safety with ticagrelor in patients with prior myocardial infarction in the approved European label: insights from PEGASUS‐TIMI 54. Eur Heart J Cardiovasc Pharmacother. 2019;5:200–206. - PMC - PubMed
-
- Genereux P, Giustino G, Witzenbichler B, Weisz G, Stuckey TD, Rinaldi MJ, Neumann FJ, Metzger DC, Henry TD, Cox DA, et al. Incidence, predictors, and impact of post‐discharge bleeding after percutaneous coronary intervention. J Am Coll Cardiol. 2015;66:1036–1045. - PubMed
-
- Bonaca MP, Bhatt DL, Braunwald E, Cohen M, Steg PG, Storey RF, Held P, Jensen EC, Sabatine MS. Design and rationale for the prevention of cardiovascular events in patients with prior heart attack using ticagrelor compared to placebo on a background of aspirin‐thrombolysis in myocardial infarction 54 (PEGASUS‐TIMI 54) trial. Am Heart J. 2014;167(437–444):e5. - PubMed
-
- Muller HG, Wang JL. Hazard rates estimation under random censoring with varying kernels and bandwidths. Biometrics. 1994;50:61–76. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

