Engineering Three-Dimensional Vascularized Cardiac Tissues
- PMID: 33559514
- PMCID: PMC9063162
- DOI: 10.1089/ten.TEB.2020.0343
Engineering Three-Dimensional Vascularized Cardiac Tissues
Abstract
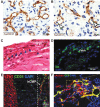
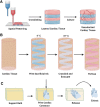
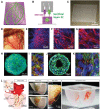
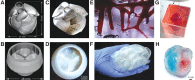
Heart disease is one of the largest burdens to human health worldwide and has very limited therapeutic options. Engineered three-dimensional (3D) vascularized cardiac tissues have shown promise in rescuing cardiac function in diseased hearts and may serve as a whole organ replacement in the future. One of the major obstacles in reconstructing these thick myocardial tissues to a clinically applicable scale is the integration of functional vascular networks capable of providing oxygen and nutrients throughout whole engineered constructs. Without perfusion of oxygen and nutrient flow throughout the entire engineered tissue not only is tissue viability compromised, but also overall tissue functionality is lost. There are many supporting technologies and approaches that have been developed to create vascular networks such as 3D bioprinting, co-culturing hydrogels, and incorporation of soluble angiogenic factors. In this state-of-the-art review, we discuss some of the most current engineered vascular cardiac tissues reported in the literature and future directions in the field. Impact statement The field of cardiac tissue engineering is rapidly evolving and is now closer than ever to having engineered tissue models capable of predicting preclinical responses to therapeutics, modeling diseases, and being used as a means of rescuing cardiac function following injuries to the native myocardium. However, a major obstacle of engineering thick cardiac tissue remains to be the integration of functional vasculature. In this review, we highlight seminal and recently published works that have influenced and pushed the field of cardiac tissue engineering toward achieving vascularized functional tissues.
Keywords: 3D printed vasculature; angiogenesis; cardiac patch; engineered cardiac tissue; regenerative medicine; vascularized cardiac tissues.
Conflict of interest statement
D.-H.K. is a scientific founder and equity holder of Curi Bio. The other authors report no conflicts.
Figures


References
-
- World Health Organization. Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2016.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

