Homogeneous batch micro-crystallization of proteins from ammonium sulfate
- PMID: 33559608
- PMCID: PMC7869895
- DOI: 10.1107/S2059798320015454
Homogeneous batch micro-crystallization of proteins from ammonium sulfate
Abstract
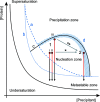
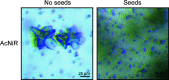
The emergence of X-ray free-electron lasers has led to the development of serial macromolecular crystallography techniques, making it possible to study smaller and more challenging crystal systems and to perform time-resolved studies on fast time scales. For most of these studies the desired crystal size is limited to a few micrometres, and the generation of large amounts of nanocrystals or microcrystals of defined size has become a bottleneck for the wider implementation of these techniques. Despite this, methods to reliably generate microcrystals and fine-tune their size have been poorly explored. Working with three different enzymes, L-aspartate α-decarboxylase, copper nitrite reductase and copper amine oxidase, the precipitating properties of ammonium sulfate were exploited to quickly transition from known vapour-diffusion conditions to reproducible, large-scale batch crystallization, circumventing the tedious determination of phase diagrams. Furthermore, the specific ammonium sulfate concentration was used to fine-tune the crystal size and size distribution. Ammonium sulfate is a common precipitant in protein crystallography, making these findings applicable to many crystallization systems to facilitate the production of large amounts of microcrystals for serial macromolecular crystallography experiments.
Keywords: ammonium sulfate; batch crystallization; microcrystals; serial crystallography.
open access.
Figures


References
-
- Albert, A., Dhanaraj, V., Genschel, U., Khan, G., Ramjee, M. K., Pulido, R., Sibanda, B. L., von Delft, F., Witty, M., Blundell, T. L., Smith, A. G. & Abell, C. (1998). Nat. Struct. Biol. 5, 289–293. - PubMed
-
- Asakura, S. & Oosawa, F. (1958). J. Polym. Sci. 33, 183–192.
-
- Barends, T. R. M., Foucar, L., Ardevol, A., Nass, K., Aquila, A., Botha, S., Doak, R. B., Falahati, K., Hartmann, E., Hilpert, M., Heinz, M., Hoffmann, M. C., Köfinger, J., Koglin, J. E., Kovacsova, G., Liang, M., Milathianaki, D., Lemke, H. T., Reinstein, J., Roome, C. M., Shoeman, R. L., Williams, G. J., Burghardt, I., Hummer, G., Boutet, S. & Schlichting, I. (2015). Science, 350, 445–450. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

