Heterogeneity of murine periosteum progenitors involved in fracture healing
- PMID: 33560227
- PMCID: PMC7906599
- DOI: 10.7554/eLife.58534
Heterogeneity of murine periosteum progenitors involved in fracture healing
Abstract
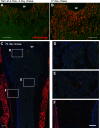
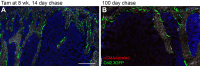
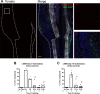
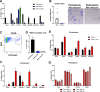
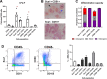
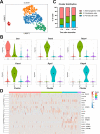
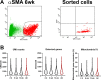
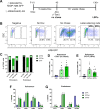
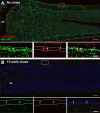
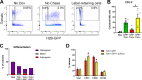
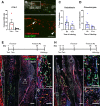
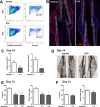
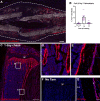
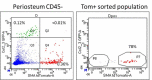
The periosteum is the major source of cells involved in fracture healing. We sought to characterize progenitor cells and their contribution to bone fracture healing. The periosteum is highly enriched with progenitor cells, including Sca1+ cells, fibroblast colony-forming units, and label-retaining cells compared to the endosteum and bone marrow. Using lineage tracing, we demonstrate that alpha smooth muscle actin (αSMA) identifies long-term, slow-cycling, self-renewing osteochondroprogenitors in the adult periosteum that are functionally important for bone formation during fracture healing. In addition, Col2.3CreER-labeled osteoblast cells contribute around 10% of osteoblasts but no chondrocytes in fracture calluses. Most periosteal osteochondroprogenitors following fracture can be targeted by αSMACreER. Previously identified skeletal stem cell populations were common in periosteum but contained high proportions of mature osteoblasts. We have demonstrated that the periosteum is highly enriched with skeletal progenitor cells, and there is heterogeneity in the populations of cells that contribute to mature lineages during periosteal fracture healing.
Keywords: alpha smooth muscle actin; fracture; mouse; osteoblast; pdgfra; periosteum; regenerative medicine; skeletal stem cell; stem cells.
© 2021, Matthews et al.
Conflict of interest statement
BM, SN, FS, JF, YC, EB, DG, IK No competing interests declared
Figures




References
-
- Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank AM, Bocian C, Woelk L, Fan H, Logan DW, Schürmann A, Saraiva LR, Schulz TJ. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem Cell-Based hematopoietic and bone regeneration. Cell Stem Cell. 2017;20:771–784. doi: 10.1016/j.stem.2017.02.009. - DOI - PMC - PubMed
-
- Bolisetty MT, Stitzel ML, Robson P. CellView: interactive exploration of high dimensional single cell RNA-seq data. bioRxiv. 2017 doi: 10.1101/123810. - DOI
Publication types
MeSH terms
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

