Measurable residual disease in elderly acute myeloid leukemia: results from the PETHEMA-FLUGAZA phase 3 clinical trial
- PMID: 33560390
- PMCID: PMC7876892
- DOI: 10.1182/bloodadvances.2020003195
Measurable residual disease in elderly acute myeloid leukemia: results from the PETHEMA-FLUGAZA phase 3 clinical trial
Abstract
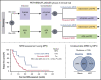
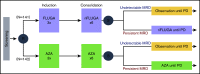
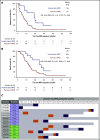
The value of measurable residual disease (MRD) in elderly patients with acute myeloid leukemia (AML) is inconsistent between those treated with intensive vs hypomethylating drugs, and unknown after semi-intensive therapy. We investigated the role of MRD in refining complete remission (CR) and treatment duration in the phase 3 FLUGAZA clinical trial, which randomized 283 elderly AML patients to induction and consolidation with fludarabine plus cytarabine (FLUGA) vs 5-azacitidine. After consolidation, patients continued treatment if MRD was ≥0.01% or stopped if MRD was <0.01%, as assessed by multidimensional flow cytometry (MFC). On multivariate analysis including genetic risk and treatment arm, MRD status in patients achieving CR (N = 72) was the only independent prognostic factor for relapse-free survival (RFS) (HR, 3.45; P = .002). Achieving undetectable MRD significantly improved RFS of patients with adverse genetics (HR, 0.32; P = .013). Longer overall survival was observed in patients with undetectable MRD after induction though not after consolidation. Although leukemic cells from most patients displayed phenotypic aberrancies vs their normal counterpart (N = 259 of 265), CD34 progenitors from cases with undetectable MRD by MFC carried extensive genetic abnormalities identified by whole-exome sequencing. Interestingly, the number of genetic alterations significantly increased from diagnosis to MRD stages in patients treated with FLUGA vs 5-azacitidine (2.2-fold vs 1.1-fold; P = .001). This study supports MRD assessment to refine CR after semi-intensive therapy or hypomethylating agents, but unveils that improved sensitivity is warranted to individualize treatment and prolong survival of elderly AML patients achieving undetectable MRD.
© 2021 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: B.P. declares honoraria for lectures from, and membership on advisory boards with, Amgen, Bristol Myers Squibb (BMS), Celgene, Janssen, Merck, Novartis, Roche, and Sanofi; unrestricted grants from Celgene, EngMab, Sanofi, and Takeda; and consultancy for Celgene, Janssen, Sanofi, and Takeda. M.T. declares honoraria for lectures from Celgene, Pfizer, Novartis, Janssen, Merck Sharp & Dohme (MSD), Daiichi, and Servier SL, and membership on advisory boards with Celgene, Novartis, Roche, and Astellas. J.S. declares honoraria for lectures, and membership on advisory boards with, Daiichi Sankyo, Pfizer, Celgene, Novartis, Roche, and Amgen. F.R. declares travel grants from Celgene, Novartis, Amgen, AbbVie, Janssen, Roche, MSD, and Daiichi Sankyo; consulting fees from Celgene, Novartis, Amgen, and AbbVie; and advisory board membership with, as well as research grants from, Celgene. E.L. declares honoraria for lectures from Janssen and Novartis, and advisory board membership with Janssen, Celgene, Astellas, and Amgen. M.-B.V. declares honoraria for lectures from, and membership on advisory boards with, Janssen, BMS, Novartis, Roche, Astellas Pharma, and Jazz Pharmaceuticals. J.L. declares travel grants from Celgene, Takeda, and Gilead. C.G. declares honoraria for lectures from Celgene, Amgen, Janssen, and Pfizer, and advisory board membership with Celgene. J.M.-L. declares honoraria for lectures from, and membership on advisory boards with, Janssen, BMS, Sanofi, Novartis, Incyte, Roche, and Amgen; and membership on the boards of directors of Hosea and Altum Sequencing. J.F.S.-M. reports consultancy for BMS, Celgene, Novartis, Takeda, Amgen, MSD, Janssen, and Sanofi, and membership on the board of directors of, or advisory committees with, Takeda. M.Á.S. declares a consulting or advisory role for Teva, Daiichi Sankyo, Orsenix, AbbVie, Novartis, and Pfizer. P. Montesinos declares advisory board and speaker’s bureau membership with, as well as research support from, AbbVie, Janssen, Novartis, Pfizer, and Teva; research support, being a consultant for, and speaker’s bureau and advisory board membership with Astellas, Celgene, and Daiichi Sankyo; being a consultant for Agios, Tolero Pharmaceutical, Glycomimetics, and Forma Therapeutics; speaker’s bureau and advisory board membership with Incyte; and research support from, and advisory board membership with, Karyopharm. The remaining authors declare no competing financial interests.
Figures
References
-
- Juliusson G, Antunovic P, Derolf A, et al. . Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113(18):4179-4187. - PubMed
-
- Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136-1152. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

