Functional Diversification, Redundancy, and Epistasis among Paralogs of the Drosophila melanogaster Obp50a-d Gene Cluster
- PMID: 33560417
- PMCID: PMC8097280
- DOI: 10.1093/molbev/msab004
Functional Diversification, Redundancy, and Epistasis among Paralogs of the Drosophila melanogaster Obp50a-d Gene Cluster
Abstract
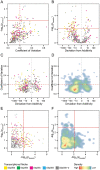
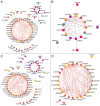
Large multigene families, such as the insect odorant-binding proteins (OBPs), are thought to arise through functional diversification after repeated gene duplications. Whereas many OBPs function in chemoreception, members of this family are also expressed in tissues outside chemosensory organs. Paralogs of the Obp50 gene cluster are expressed in metabolic and male reproductive tissues, but their functions and interrelationships remain unknown. Here, we report the genetic dissection of four members of the Obp50 cluster, which are in close physical proximity without intervening genes. We used CRISPR technology to excise the entire cluster while introducing a PhiC31 reintegration site to reinsert constructs in which different combinations of the constituent Obp genes were either intact or rendered inactive. We performed whole transcriptome sequencing and assessed sexually dimorphic changes in transcript abundances (transcriptional niches) associated with each gene-edited genotype. Using this approach, we were able to estimate redundancy, additivity, diversification, and epistasis among Obp50 paralogs. We analyzed the effects of gene editing of this cluster on organismal phenotypes and found a significant skewing of sex ratios attributable to Obp50a, and sex-specific effects on starvation stress resistance attributable to Obp50d. Thus, there is functional diversification within the Obp50 cluster with Obp50a contributing to development and Obp50d to stress resistance. The deletion-reinsertion approach we applied to the Obp50 cluster provides a general paradigm for the genetic dissection of paralogs of multigene families.
Keywords: RNAseq; evolutionary genetics; functional diversification; multigene families; odorant-binding proteins; transcriptional niche.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution.
Figures




References
-
- Aguila JR, Suszko J, Gibbs AG, Hoshizaki DK.. 2007. The role of larval fat cells in adult Drosophila melanogaster. J Exp Biol. 210(6):956–963. - PubMed
-
- Bischof J, Björklund M, Furger E, Schertel C, Taipale J, Basler K.. 2013. A versatile platform for creating a comprehensive UAS-ORFeome library in Drosophila. Development 140(11):2434–2442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

