Antidepressants for functional abdominal pain disorders in children and adolescents
- PMID: 33560523
- PMCID: PMC8094232
- DOI: 10.1002/14651858.CD008013.pub3
Antidepressants for functional abdominal pain disorders in children and adolescents
Abstract
Background: Functional Abdominal Pain Disorders (FAPDs) present a considerable burden to paediatric patients, impacting quality of life, school attendance and causing higher rates of anxiety and depression disorders. There are no international guidelines for the management of this condition. A previous Cochrane Review in 2011 found no evidence to support the use of antidepressants in this context.
Objectives: To evaluate the current evidence for the efficacy and safety of antidepressants for FAPDs in children and adolescents.
Search methods: In this updated review, we searched the Cochrane Library, PubMed, MEDLINE, Embase, PsycINFO and two clinical trial registers from inception until 03 February 2020. We also updated our search of databases of ongoing research, reference lists and 'grey literature' from inception to 03 February 2020.
Selection criteria: We included randomised controlled trials (RCTs) comparing antidepressants to placebo, to no treatment or to any other intervention, in children aged 4 to 18 years with a FAPD diagnosis as per the Rome or any other defined criteria (as defined by the authors). The primary outcomes of interest included treatment success (as defined by the authors), pain severity, pain frequency and withdrawal due to adverse events.
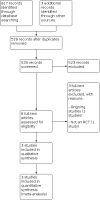
Data collection and analysis: Two review authors checked all citations independently, resolving disagreement with a third-party arbiter. We reviewed all potential studies in full text, and once again made independent decisions, with disagreements resolved by consensus. We conducted data extraction and 'Risk of bias' assessments independently, following Cochrane methods. Where homogeneous data were available, we performed meta-analysis using a random-effects model. We conducted GRADE analysis.
Main results: We found one new study in this updated search, making a total of three trials (223 participants) eligible for inclusion: two using amitriptyline (AMI) and one using citalopram. For the primary outcome of treatment success, two studies used reports of success on a symptom-based Likert scale, with either a two-point reduction or the two lowest levels defined as success. The third study defined success as a 15% improvement in quality of life (QOL) ratings scales. Therefore, meta-analysis did not include this final study due to the heterogeneity of the outcome measure. There is low-certainty evidence that there may be no difference when antidepressants are compared with placebo (risk ratio (RR) 1.17, 95% confidence interval (CI) 0.87 to 1.56; 2 studies, 205 participants; I2 = 0%). We downgraded the evidence for significant imprecision due to extremely sparse data (see Summary of findings table 1). The third study reported that participants receiving antidepressants were significantly more likely than those receiving placebo to experience at least a 15% improvement in overall QOL score at 10 and 13 weeks (P = 0.007 and P = 0.002, respectively (absolute figures were not given)). The analysis found no difference in withdrawals due to adverse events between antidepressants and placebo: RR 3.17 (95% CI 0.65 to 15.33), with very low certainty due to high risk of bias in studies and imprecision due to low event and participant numbers. Sensitivity analysis using a fixed-effect model and analysing just for AMI found no change in this result. Due to heterogeneous and limited reporting, no further meta-analysis was possible.
Authors' conclusions: There may be no difference between antidepressants and placebo for treatment success of FAPDs in childhood. There may be no difference in withdrawals due to adverse events, but this is also of low certainty. There is currently no evidence to support clinical decision making regarding the use of these medications. Further studies must consider sample size, homogenous and relevant outcome measures and longer follow up.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
MB: Consultant for Shire, Norgine, Coloplast, Danone, Takeda, Allergan, Shire, FrieslandCampina, United Pharamceuticals.
MT: None known
MG: Since August 2016, I have received travel fees to attend international scientific and training meetings from Pharma companies. These grants included no honoraria, inducement, advisory role or any other relationship and were restricted to the travel and meeting related costs of attending such meetings. These include: DDW May 2017, World Congress of Gastroenterology October 2017, DDW May 2018, Advances in IBD December 2018, DDW May 2019. None of these companies have had any involvement in any works completed by me and I have never had any payments for any other activities for them, as confirmed below. From this date onwards, I have made a personal undertaking to take no further funds from any pharmaceutical or formula company in any form for travel or other related activities. This is to lift the limitations such funding has on my ability to act as a first and corresponding author on reviews, in line with the Cochrane policies on such matters and is reported in line with these policies. These current declarations will expire over the next three years and this statement updated regularly to reflect this. RR: None known.
CB: None known.
Figures




Update of
-
Antidepressants for the treatment of abdominal pain-related functional gastrointestinal disorders in children and adolescents.Cochrane Database Syst Rev. 2011 Jul 6;2011(7):CD008013. doi: 10.1002/14651858.CD008013.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2021 Feb 9;2:CD008013. doi: 10.1002/14651858.CD008013.pub3. PMID: 21735420 Free PMC article. Updated.
References
References to studies included in this review
Bahar 2008 {published data only}
-
- Bahar RJ, Collins BS, Steinmetz B, Ament ME. Double-blind placebo-controlled trial of amitriptyline for the treatment of irritable bowel syndrome in adolescents. Journal of Pediatrics 2008;152(2):685-9. [PMID: ] - PubMed
Roohafza 2014 {published data only}
-
- Roohafza H, Pourmoghaddas H, Sanien H, Gholamrezaei A. Citalopram for pediatric functional abdominal pain: a randomized, placebo-controlled trial. Neurogastroenterology and Motility 2014;26(11):1642-50. - PubMed
References to studies excluded from this review
Campo 2004 {published data only}
-
- Campo JV, Perel J, Lucas A, Bridge J, Ehmann M, Kalas C, et al. Citalopram treatment of pediatric recurrent abdominal pain and comorbid internalizing disorders: an exploratory study. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(10):1234-42. [PMID: ] - PubMed
References to ongoing studies
ACTRN12613000158763 {published data only}
-
- Evaluation treatment efficacy of citalopram, synbiotic, and mebeverine for children with functional abdominal pain; a randomized, placebo-controlled study.. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=1261... Date first registered 11/2/13.
CTRI/2018/08/015365 {published data only}
-
- Randomized placebo-controlled trial with amitriptyline in children with functional abdominal pain disorders (FAPD). http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=27840&EncHid... First registered 16/8/18.
Additional references
Apley 1958
Assa 2015
-
- Assa A, Ish-Tov A, Rinawi F, Shamir R. School attendance in children with functional abdominal pain and inflammatory bowel diseases. Journal of Pediatric Gastroenterology and Nutrition 2015;61(5):553-7. - PubMed
Atkins 2004
Black 2020
-
- Black CJ, Yuan Y, Selinger CP, Camilleri M, Quigley EM, Moayyedi P, Ford AC. Efficacy of soluble fibre, antispasmodic drugs, and gut-brain neuromodulators in irritable bowel syndrome: a systematic review and network meta-analysis. Lancet Gastroenterology and Hepatology 2020;5(2):117-31. - PubMed
Borenstein 2009
-
- Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to Meta-Analysis. Chichester, West Sussex, UK: John Wiley & Sons Ltd, 2009.
Boylan 2020
-
- Boylan K, MacQueen G, Kirkpatrick R, Lee J, Santaguida PL. A systematic review of interventions for treatment resistant major depressive disorder in adolescents. European Child & Adolescent Psychiatry 2020;29(4):433-443. - PubMed
Cohen 2010
-
- Cohen RD. Systematic review: the costs of ulcerative colitis in Western countries. Alimentary Pharmaology and Therapeutics 2010;31(7):693-707. - PubMed
Cooper 2017
Deeks 2020
-
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated July 2019). The Cochrane Collaboration, 2020. Available from www.training.cochrane.org/handbook.
Drossman 2006
-
- Drossman DA. Rome III: the new criteria. Chinese Journal of Digestive Diseases 2006;7(4):181-5. [PMID: ] - PubMed
Drossman 2016
-
- Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology 2016;150(16):1262-79. - PubMed
Drossman 2018
-
- Drossman DA, Tack J, Ford AC, Szigethy E, Törnblom H, Van Oudenhove L. Neuromodulators for functional gastrointestinal disorders (disorders of gut-brain interaction): a Rome Foundation Working Team report. Gastroenterology 2018;154(4):1140-71. - PubMed
Ford 2018
-
- Ford AC, Moayyedi P, Chey WD, Harris LA, Lacy BE, Saito YA, et al, ACG Task Force on Management of Irritable Bowel Syndrome. American College of Gastroenterology Monograph on Management of Irritable Bowel Syndrome. American Journal of Gastroenterology 2018;113(Suppl 2):1-18. - PubMed
Furukawa 2019
Gartlehner 2011
-
- Gartlehner G, Hansen RA, Morgan LC, Thaler K, Lux LJ, Van Noord M, et al. Second-generation antidepressants in the pharmacologic treatment of adult depression: an update of the 2007 comparative effectiveness review. www.ncbi.nlm.nih.gov/books/NBK83442/pdf/Bookshelf_NBK83442.pdf 2011. [Report No: 12-EHC012-EF] - PubMed
Geoffroy 2019
-
- Geoffroy PA, Schroder CM, Reynaud E, Bourgin P. Efficacy of light therapy versus antidepressant drugs, and of the combination versus monotherapy, in major depressive episodes: A systematic review and meta-analysis. Sleep Medicine Reviews 2019;48:101213. - PubMed
Gieteling 2008
-
- Gieteling MJ, Bierma-Zeinstra SM, Passchier J, Berger MY. Prognosis of chronic or recurrent abdominal pain in children. Journal of Pediatric Gastroenterology and Nutrition 2008;47(3):316-26. [PMID: ] - PubMed
Guyatt 2011
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. Introduction—GRADE evidence profiles and summary of findings tables.. Journal of Clinical Epidemiology 2011;64:383-94. - PubMed
Higgins 2011
-
- Higgins JP, Altman DA, Sterne JA, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2020
-
- Higgins JP, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). The Cochrane Collaboration, 2020. Available from www.training.cochrane.org/handbook.
Hyams 2016
-
- Hyams JS, Di Lorenzo C, Saps M, Shulman RJ, Staiano A, Van Tilburg M. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology 2016;150(6):1456-68.
Kaminski 2012
-
- Kaminski A, Kamper A, Thaler K, Chapman A, Gartlehner G. Antidepressants for the treatment of abdominal pain-relatedfunctional gastrointestinal disorders in children and adolescents. Cochrane Database of Systematic Reviews 2011, Issue 7. Art. No: CD008013. [DOI: 10.1002/14651858.CD008013.pub2] - DOI - PMC - PubMed
Korterink 2015
Martin 2017
Newton 2019
-
- Newton E, Schosheim A, Patel S, Chitkara DK, Van Tilburg MA. The role of psychological factors in pediatric functional abdominal pain disorders. Neurogastroenterology and Motility 2019;31(6):e13538. - PubMed
Ogawa 2019
Page 2020
-
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). The Cochrane Collaboration, 2020. Available from www.training.cochrane.org/handbook.
Pate 2020
-
- Pate JW, Hancock MJ, Hush JM, Gray K, Pounder M, Pacey V. Prognostic factors for pain and functional disability in children and adolescents with persisting pain: a systematic review and meta-analysis. European Journal of Pain 2020;24(4):722-41. - PubMed
Patrick 1997
-
- Patrick DL, Drossman DA, Ihunnaya OF. user’s manual and scoring diskette for United States version. Seattle: University of Washington, 1997.
Rexwinkel 2019
-
- Rexwinkel R, Zeevenhooven J, Van Etten-Jamuludin FS, Benninga MA, Tabbers MM. Side effects associated with pharmacotherapy for pediatric irritable bowel syndrome and functional abdominal pain - not otherwise specified: a systematic review. Expert Opinion on Drug Safety 2019;18(2):111-25. - PubMed
Ruepert 2011
-
- Ruepert L, Quartero AO, Wit NJ, Van der Heijden GJ, Rubin G, Muris JW. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database of Systematic Reviews 2011, Issue 8. Art. No: CD003460. [DOI: 10.1002/14651858.CD003460.pub3] - DOI - PMC - PubMed
Santucci 2020
-
- Santucci NR, Saps M, Van Tilburg MA. New advances in the treatment of paediatric functional abdominal pain disorders. Lancet Gastroenterology and Hepatology 2020;5(3):316-28. - PubMed
Saps 2016
-
- Saps M, Van Tilburg MA, Lavigne JV, Miranda A, Benninga MA, Taminiau JA, et al. Recommendations for pharmacological clinical trials in children with irritable bowel syndrome: the Rome foundation pediatric subcommittee on clinical trials. Neurogastroenterology and Motility 2016;28(11):1619-31. - PubMed
Saps 2018
-
- The Rome foundation pediatric subcommittee on clinical trials Saps M, Lavigne JV, Tilburg MA, Miranda A, Benninga MA, Taminiau JA, Di Lorenzo C. Endpoints, reliability, and meaningful changes in clinical trials for children with irritable bowel syndrome.. Neurogastroenterol Motil 2018 May;30(5):13308. - PubMed
Schünemann 2020
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). The Cochrane Collaboration, 2020. Available from www.training.cochrane.org/handbook.
Schurman 2010
-
- Schurman JV, Hunter HL, Friesen CA. Conceptualization and treatment of chronic abdominal pain in pediatric gastroenterology practice. Journal of Pediatric Gastroenterology and Nutrition 2010;50(1):32-7. - PubMed
Smith 1997
-
- Smith GA, Strausbaugh SD, Harbeck-Weber C, Cohen DM, Shields BJ, PowersJD. New non-cocaine-containing topical anesthetics compared with tetracaine-adrenaline-cocaine during repair of lacerations.. Pediatrics 1997;100:825-30. - PubMed
Varni 2015
-
- Varni JW, Bendo CB, Nurko S, Shulman RJ, Self MM, Franciosi JP, et al. Health-related quality of life in pediatric patients with functional and organic gastrointestinal diseases. Journal of Pediatriatrics 2015;166(1):85-90. - PubMed
Zar‐Kessler 2017
-
- Zar-Kessler CA, Belkind-Gerson J, Bender S, Kuo BM. Treatment of functional abdominal pain with antidepressants: benefits, adverse effects, and the gastroenterologist's role. Journal of Pediatric Gastroenterology and Nutrition 2017;65(1):16-21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

