Primary Tumor Resection Plus Chemotherapy Versus Chemotherapy Alone for Colorectal Cancer Patients With Asymptomatic, Synchronous Unresectable Metastases (JCOG1007; iPACS): A Randomized Clinical Trial
- PMID: 33560877
- PMCID: PMC8078424
- DOI: 10.1200/JCO.20.02447
Primary Tumor Resection Plus Chemotherapy Versus Chemotherapy Alone for Colorectal Cancer Patients With Asymptomatic, Synchronous Unresectable Metastases (JCOG1007; iPACS): A Randomized Clinical Trial
Abstract
Purpose: It remains controversial whether primary tumor resection (PTR) before chemotherapy improves survival in patients with colorectal cancer (CRC) with asymptomatic primary tumor and synchronous unresectable metastases.
Patients and methods: This randomized phase III study investigated the superiority of PTR followed by chemotherapy versus chemotherapy alone in relation to overall survival (OS) in patients with unresectable stage IV asymptomatic CRC and three or fewer unresectable metastatic diseases confined to the liver, lungs, distant lymph nodes, or peritoneum. Chemotherapy regimens of either mFOLFOX6 plus bevacizumab or CapeOX plus bevacizumab were decided before study entry. The primary end point was OS, which was analyzed by intention-to-treat.
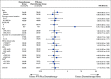
Results: Between June 2012 and September 2019, a total of 165 patients were randomly assigned to either chemotherapy alone (84 patients) or PTR plus chemotherapy (81 patients). When the first interim analysis was performed in September 2019 with 50% (114/227) of the expected events observed among 160 patients at the data cutoff date of June 5, 2019, the Data and Safety Monitoring Committee recommended early termination of the trial because of futility. With a median follow-up of 22.0 months, median OS was 25.9 months (95% CI, 19.9 to 31.5) in the PTR plus chemotherapy arm and 26.7 (95% CI, 21.9 to 32.5) in the chemotherapy-alone arm (hazard ratio, 1.10; 95% CI, 0.76 to 1.59; one-sided P = .69). Three postoperative deaths occurred in the PTR plus chemotherapy arm.
Conclusion: Given that PTR followed by chemotherapy showed no survival benefit over chemotherapy alone, PTR should no longer be considered a standard of care for patients with CRC with asymptomatic primary tumors and synchronous unresectable metastases.
Figures

Comment in
-
Primary Tumor Resection in Colorectal Cancer With Synchronous Unresectable Metastasis: Time to End the Debate?J Clin Oncol. 2021 Apr 1;39(10):1095-1097. doi: 10.1200/JCO.21.00057. Epub 2021 Feb 16. J Clin Oncol. 2021. PMID: 33591837 No abstract available.
-
Reply to B. Bozkurt Duman et al.J Clin Oncol. 2021 Sep 10;39(26):2970-2971. doi: 10.1200/JCO.21.00967. Epub 2021 Jun 2. J Clin Oncol. 2021. PMID: 34236894 No abstract available.
-
Do the Survival Data of Primary Tumor Resection Provide Sufficient Data Without Considering the Tumor Sidedness, Predictive Biomarkers, and Biologic Agents?J Clin Oncol. 2021 Sep 10;39(26):2970. doi: 10.1200/JCO.21.00560. Epub 2021 Jun 2. J Clin Oncol. 2021. PMID: 34236898 No abstract available.
-
Primary Tumor Resection in Colorectal Cancer with Unresectable Synchronous Metastasis: Time to Reconsider the Role of the Surgeon.Ann Surg Oncol. 2022 Jan;29(1):1-3. doi: 10.1245/s10434-021-10949-4. Epub 2021 Oct 20. Ann Surg Oncol. 2022. PMID: 34671880 No abstract available.
-
Patient with unresectable colorectal liver metastases and asymptomatic primary tumor: end of the debate!Hepatobiliary Surg Nutr. 2022 Jun;11(3):412-414. doi: 10.21037/hbsn-22-176. Hepatobiliary Surg Nutr. 2022. PMID: 35693410 Free PMC article. No abstract available.
References
-
- Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet 383: 1490-1502, 2014 - PubMed
-
- Sarela AI Guthrie JA Seymour MT, et al. : Non-operative management of the primary tumor in patients with incurable stage IV colorectal cancer. Br J Surg 88:1352-1356, 2001 - PubMed
-
- Galizia G Lieto E Orditura M, et al. : First-line chemo- therapy vs bowel tumor resection plus chemotherapy for patients with unresectable synchronous colorectal hepatic metastases. Arch Surg 143:352-358, 2008 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

