Sensitive tracking of circulating viral RNA through all stages of SARS-CoV-2 infection
- PMID: 33561010
- PMCID: PMC8011898
- DOI: 10.1172/JCI146031
Sensitive tracking of circulating viral RNA through all stages of SARS-CoV-2 infection
Abstract
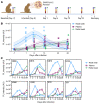
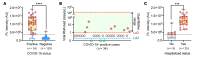
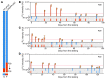
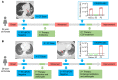
BACKGROUNDCirculating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA may represent a more reliable indicator of infection than nasal RNA, but quantitative reverse transcription PCR (RT-qPCR) lacks diagnostic sensitivity for blood samples.METHODSA CRISPR-augmented RT-PCR assay that sensitively detects SARS-CoV-2 RNA was employed to analyze viral RNA kinetics in longitudinal plasma samples from nonhuman primates (NHPs) after virus exposure; to evaluate the utility of blood SARS-CoV-2 RNA detection for coronavirus disease 2019 (COVID-19) diagnosis in adults cases confirmed by nasal/nasopharyngeal swab RT-PCR results; and to identify suspected COVID-19 cases in pediatric and at-risk adult populations with negative nasal swab RT-qPCR results. All blood samples were analyzed by RT-qPCR to allow direct comparisons.RESULTSCRISPR-augmented RT-PCR consistently detected SARS-CoV-2 RNA in the plasma of experimentally infected NHPs from 1 to 28 days after infection, and these increases preceded and correlated with rectal swab viral RNA increases. In a patient cohort (n = 159), this blood-based assay demonstrated 91.2% diagnostic sensitivity and 99.2% diagnostic specificity versus a comparator RT-qPCR nasal/nasopharyngeal test, whereas RT-qPCR exhibited 44.1% diagnostic sensitivity and 100% specificity for the same blood samples. This CRISPR-augmented RT-PCR assay also accurately identified patients with COVID-19 using one or more negative nasal swab RT-qPCR results.CONCLUSIONResults of this study indicate that sensitive detection of SARS-CoV-2 RNA in blood by CRISPR-augmented RT-PCR permits accurate COVID-19 diagnosis, and can detect COVID-19 cases with transient or negative nasal swab RT-qPCR results, suggesting that this approach could improve COVID-19 diagnosis and the evaluation of SARS-CoV-2 infection clearance, and predict the severity of infection.TRIAL REGISTRATIONClinicalTrials.gov. NCT04358211.FUNDINGDepartment of Defense, National Institute of Allergy and Infectious Diseases, National Institute of Child Health and Human Development, and the National Center for Research Resources.
Keywords: COVID-19; Diagnostics; Molecular diagnosis.
Conflict of interest statement
Figures
Similar articles
-
Low level SARS-CoV-2 RNA detected in plasma samples from a cohort of Nigerians: Implications for blood transfusion.PLoS One. 2021 Jun 10;16(6):e0252611. doi: 10.1371/journal.pone.0252611. eCollection 2021. PLoS One. 2021. PMID: 34111179 Free PMC article.
-
CRISPR-based assay reveals SARS-CoV-2 RNA dynamic changes and redistribution patterns in non-human primate model.Emerg Microbes Infect. 2022 Dec;11(1):629-638. doi: 10.1080/22221751.2022.2038020. Emerg Microbes Infect. 2022. PMID: 35108153 Free PMC article.
-
Comparison of SARS-CoV-2 PCR-Based Detection Using Saliva or Nasopharyngeal Swab Specimens in Asymptomatic Populations.Microbiol Spectr. 2021 Sep 3;9(1):e0006221. doi: 10.1128/Spectrum.00062-21. Epub 2021 Aug 25. Microbiol Spectr. 2021. PMID: 34431689 Free PMC article.
-
Ravaging SARS-CoV-2: rudimentary diagnosis and puzzling immunological responses.Curr Med Res Opin. 2021 Feb;37(2):207-217. doi: 10.1080/03007995.2020.1862532. Epub 2020 Dec 26. Curr Med Res Opin. 2021. PMID: 33306409 Free PMC article. Review.
-
Detection profile of SARS-CoV-2 using RT-PCR in different types of clinical specimens: A systematic review and meta-analysis.J Med Virol. 2021 Feb;93(2):719-725. doi: 10.1002/jmv.26349. Epub 2020 Aug 2. J Med Virol. 2021. PMID: 32706393 Free PMC article.
Cited by
-
Accuracy of clustered regularly interspaced short palindromic repeats (CRISPR) to diagnose COVID-19, a meta-analysis.Microb Pathog. 2022 Apr;165:105498. doi: 10.1016/j.micpath.2022.105498. Epub 2022 Mar 25. Microb Pathog. 2022. PMID: 35341958 Free PMC article.
-
Biologic correlates of beneficial convalescent plasma therapy in a COVID-19 patient reveal disease resolution mechanisms.medRxiv [Preprint]. 2022 Feb 3:2022.02.03.22269612. doi: 10.1101/2022.02.03.22269612. medRxiv. 2022. Update in: Front Med (Lausanne). 2022 Jun 15;9:915367. doi: 10.3389/fmed.2022.915367. PMID: 35132422 Free PMC article. Updated. Preprint.
-
Longitudinal Analysis of Biologic Correlates of COVID-19 Resolution: Case Report.Front Med (Lausanne). 2022 Jun 15;9:915367. doi: 10.3389/fmed.2022.915367. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35783607 Free PMC article.
-
Plasma SARS-CoV-2 nucleocapsid antigen levels are associated with progression to severe disease in hospitalized COVID-19.Crit Care. 2022 Sep 14;26(1):278. doi: 10.1186/s13054-022-04153-3. Crit Care. 2022. PMID: 36104754 Free PMC article.
-
CRISPR detection of circulating cell-free Mycobacterium tuberculosis DNA in adults and children, including children with HIV: a molecular diagnostics study.Lancet Microbe. 2022 Jul;3(7):e482-e492. doi: 10.1016/S2666-5247(22)00087-8. Epub 2022 May 31. Lancet Microbe. 2022. PMID: 35659882 Free PMC article.
References
-
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/ Updated January 29, 2021. Accessed February 9, 2021.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

