Augmented antibody-based anticancer therapeutics boost neutrophil cytotoxicity
- PMID: 33561014
- PMCID: PMC7954609
- DOI: 10.1172/JCI134680
Augmented antibody-based anticancer therapeutics boost neutrophil cytotoxicity
Abstract
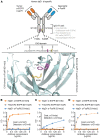
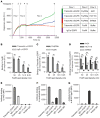
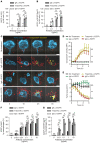
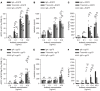
Most clinically used anticancer mAbs are of the IgG isotype, which can eliminate tumor cells through NK cell-mediated antibody-dependent cellular cytotoxicity and macrophage-mediated antibody-dependent phagocytosis. IgG, however, ineffectively recruits neutrophils as effector cells. IgA mAbs induce migration and activation of neutrophils through the IgA Fc receptor (FcαRI) but are unable to activate NK cells and have poorer half-life. Here, we combined the agonistic activity of IgG mAbs and FcαRI targeting in a therapeutic bispecific antibody format. The resulting TrisomAb molecules recruited NK cells, macrophages, and neutrophils as effector cells for eradication of tumor cells in vitro and in vivo. Moreover, TrisomAb had long in vivo half-life and strongly decreased B16F10gp75 tumor outgrowth in mice. Importantly, neutrophils of colorectal cancer patients effectively eliminated tumor cells in the presence of anti-EGFR TrisomAb but were less efficient in mediating killing in the presence of IgG anti-EGFR mAb (cetuximab). The clinical application of TrisomAb may provide potential alternatives for cancer patients who do not benefit from current IgG mAb therapy.
Keywords: Immunoglobulins; Neutrophils; Therapeutics.
Conflict of interest statement
Figures
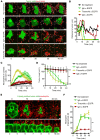
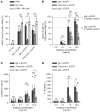
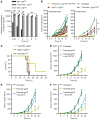
References
-
- Ferris RL, et al. Rationale for combination of therapeutic antibodies targeting tumor cells and immune checkpoint receptors: harnessing innate and adaptive immunity through IgG1 isotype immune effector stimulation. Cancer Treat Rev. 2018;63:48–60. doi: 10.1016/j.ctrv.2017.11.008. - DOI - PMC - PubMed
-
- Chanan-Khan A, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17(2):200–211. doi: 10.1016/S1470-2045(15)00465-9. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

