Internal defect scanning of sweetpotatoes using interactance spectroscopy
- PMID: 33561172
- PMCID: PMC7872240
- DOI: 10.1371/journal.pone.0246872
Internal defect scanning of sweetpotatoes using interactance spectroscopy
Abstract
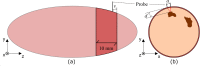
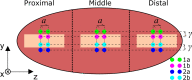
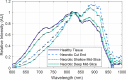
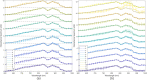
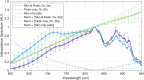
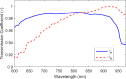
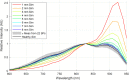
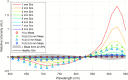
While standard visible-light imaging offers a fast and inexpensive means of quality analysis of horticultural products, it is generally limited to measuring superficial (surface) defects. Using light at longer (near-infrared) or shorter (X-ray) wavelengths enables the detection of superficial tissue bruising and density defects, respectively; however, it does not enable the optical absorption and scattering properties of sub-dermal tissue to be quantified. This paper applies visible and near-infrared interactance spectroscopy to detect internal necrosis in sweetpotatoes and develops a Zemax scattering simulation that models the measured optical signatures for both healthy and necrotic tissue. This study demonstrates that interactance spectroscopy can detect the unique near-infrared optical signatures of necrotic tissues in sweetpotatoes down to a depth of approximately 5±0.5 mm. We anticipate that light scattering measurement methods will represent a significant improvement over the current destructive analysis methods used to assay for internal defects in sweetpotatoes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures









References
-
- Qiu L, Perelman LT. Confocal Light Absorption and Scattering Spectroscopic Microscopy Tuchin VV, editor. Handbook of Photonics for Biomedical Science. Boca Raton: Crc Press-Taylor & Francis Group; 2010. pp. 465–480.
-
- Lu R, Van Beers R, Saeys W, Li C, Cen H. Measurement of optical properties of fruits and vegetables: A review. Postharvest Biology and Technology. 2020;159: UNSP 111003 10.1016/j.postharvbio.2019.111003 - DOI
-
- Noordam JC, Otten GW, Timmermans TJ, van Zwol BH. High-speed potato grading and quality inspection based on a color vision system Machine Vision Applications in Industrial Inspection VIII. International Society for Optics and Photonics; 2000. pp. 206–217.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

