Specific decorations of 17-hydroxygeranyllinalool diterpene glycosides solve the autotoxicity problem of chemical defense in Nicotiana attenuata
- PMID: 33561278
- PMCID: PMC8254506
- DOI: 10.1093/plcell/koab048
Specific decorations of 17-hydroxygeranyllinalool diterpene glycosides solve the autotoxicity problem of chemical defense in Nicotiana attenuata
Abstract
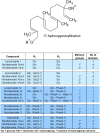
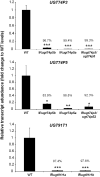
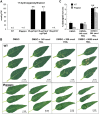
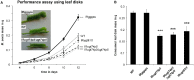
The native diploid tobacco Nicotiana attenuata produces abundant, potent anti-herbivore defense metabolites known as 17-hydroxygeranyllinalool diterpene glycosides (HGL-DTGs) whose glycosylation and malonylation biosynthetic steps are regulated by jasmonate signaling. To characterize the biosynthetic pathway of HGL-DTGs, we conducted a genome-wide analysis of uridine diphosphate glycosyltransferases (UGTs) and identified 107 family-1 UGT members. The transcript levels of three UGTs were highly correlated with the transcript levels two key HGL-DTG biosynthetic genes: geranylgeranyl diphosphate synthase (NaGGPPS) and geranyllinalool synthase (NaGLS). NaGLS's role in HGL-DTG biosynthesis was confirmed by virus-induced gene silencing. Silencing the Uridine diphosphate (UDP)-rhamnosyltransferase gene UGT91T1 demonstrated its role in the rhamnosylation of HGL-DTGs. In vitro enzyme assays revealed that UGT74P3 and UGT74P4 use UDP-glucose for the glucosylation of 17-hydroxygeranyllinalool (17-HGL) to lyciumoside I. Plants with stable silencing of UGT74P3 and UGT74P5 were severely developmentally deformed, pointing to a phytotoxic effect of the aglycone. The application of synthetic 17-HGL and silencing of the UGTs in HGL-DTG-free plants confirmed this phytotoxic effect. Feeding assays with tobacco hornworm (Manduca sexta) larvae revealed the defensive functions of the glucosylation and rhamnosylation steps in HGL-DTG biosynthesis. Glucosylation of 17-HGL is therefore a critical step that contributes to the resulting metabolites' defensive function and solves the autotoxicity problem of this potent chemical defense.
© The Author(s) 2021. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Figures





References
-
- Baker R, Parton AH, Howse PE (1982) Identification of an acyclic diterpene alcohol in the defense secretion of soldiers of Reticulitermes-Lucifugus. Experientia 38: 297–298
-
- Bubner B, Gase K, Berger B, Link D, Baldwin IT (2006) Occurrence of tetraploidy in Nicotiana attenuata plants after Agrobacterium-mediated transformation is genotype specific but independent of polysomaty of explant tissue. Plant Cell Rep 25: 668–675 - PubMed
-
- Cote F, Cormier F, Dufresne C, Willemot C (2000) Properties of a glucosyltransferase involved in crocin synthesis. Plant Sci 153: 55–63
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

