ADAMTS5 is required for normal trabeculated bone development in the mandibular condyle
- PMID: 33561540
- PMCID: PMC8759092
- DOI: 10.1016/j.joca.2021.01.005
ADAMTS5 is required for normal trabeculated bone development in the mandibular condyle
Abstract
Objective: Determine the role of the extracellular matrix protease ADAMTS5 in development of the trabeculated bone of the mandibular condyle.
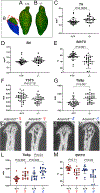
Methods: The mandibular condyles of wild type and mice deficient in the protease ADAMTS5 were examined for histopathology with Safranin O staining. Microcomputed tomography was performed to analyze the developing bone of the mandibular condyle. RNAscope and immunohistochemistry were utilized to investigate cell type and extracellular matrix expression.
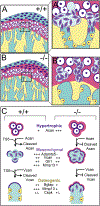
Results: Mice deficient in Adamts5, (Adamts5tm1Dgen/J) exhibit an increase in trabecular separation (n = 37 wild type; n = 27: P < 0.0001) and reduction of trabecular thickness P = 0.0116 and bone volume fraction P = 0.0869 in the mandibular condylar head compared to wild type littermates. The altered bone parameters were more pronounced in male Adamts5-/- mice compared to female Adamts5-/- mice (TbSp; P = 0.03). Adamts5 was co-expressed with versican and Gli1 in mesenchymal, stem-like cells in the transition zone where the trabeculated bone is adjacent to mature hypertrophic chondrocytes. Loss of Adamts5 caused a reduction of Bglap expressing osteoblasts throughout mandibular condylar development and in young adult mice. The protease Mmp13, that is involved in mineralization and is expressed by hypertrophic chondrocytes and osteoblasts, was reduced in the mandibular condyle of Adamts5 deficient mice.
Conclusion: This is the first report of a novel and critical role for Adamts5 in bone formation within the mandibular condyle of the temporomandibular joint. These data indicate Adamts5 may be required in the transdifferentiation of hypertrophic chondrocytes to osteoblasts during trabecular bone formation in development of the mandibular condyle.
Keywords: ADAMTS; Bone; Extracellular matrix; Mandibular condyle; Temporomandibular joint (TMJ).
Copyright © 2021 Osteoarthritis Research Society International. Published by Elsevier Ltd. All rights reserved.
Figures



References
-
- Wang XD, Zhang JN, Gan YH, Zhou YH. Current understanding of pathogenesis and treatment of TMJ osteoarthritis. J Dent Res 2015; 94: 666–673. - PubMed
-
- Milam SB. Pathogenesis of degenerative temporomandibular joint arthritides. Odontology 2005; 93: 7–15. - PubMed
-
- Mizoguchi I, Toriya N, Nakao Y. Growth of the manddible and biological characteristics of the mandibular condylar cartilage. Japanese Dental Science Review 2013; 49: 139–150.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

