Cerebrospinal fluid in COVID-19: A systematic review of the literature
- PMID: 33561753
- PMCID: PMC7833669
- DOI: 10.1016/j.jns.2021.117316
Cerebrospinal fluid in COVID-19: A systematic review of the literature
Abstract
Objective: We sought to review the literature on cerebrospinal fluid (CSF) testing in patients with COVID-19 for evidence of viral neuroinvasion by SARS-CoV-2.
Methods: We performed a systematic review of Medline and Embase between December 1, 2019 and November 18, 2020 to identify case reports or series of patients who had COVID-19 diagnosed based on positive SARS-CoV-2 polymerase chain reaction (PCR) or serologic testing and had CSF testing due to a neurologic symptom.
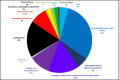
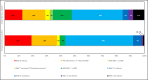
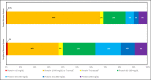
Results: We identified 242 relevant documents which included 430 patients with COVID-19 who had acute neurological symptoms prompting CSF testing. Of those, 321 (75%) patients had symptoms that localized to the central nervous system (CNS). Of 304 patients whose CSF was tested for SARS-CoV-2 PCR, there were 17 (6%) whose test was positive, all of whom had symptoms that localized to the central nervous system (CNS). The majority (13/17, 76%) of these patients were admitted to the hospital because of neurological symptoms. Of 58 patients whose CSF was tested for SARS-CoV-2 antibody, 7 (12%) had positive antibodies with evidence of intrathecal synthesis, all of whom had symptoms that localized to the CNS. Of 132 patients who had oligoclonal bands evaluated, 3 (2%) had evidence of intrathecal antibody synthesis. Of 77 patients tested for autoimmune antibodies in the CSF, 4 (5%) had positive findings.
Conclusion: Detection of SARS-CoV-2 in CSF via PCR or evaluation for intrathecal antibody synthesis appears to be rare. Most neurological complications associated with SARS- CoV-2 are unlikely to be related to direct viral neuroinvasion.
Keywords: COVID-19; Cerebrospinal fluid; Neuroinvasion; SARS-CoV-2.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
None.
Figures
Comment in
-
Fulminant Encephalitis Caused by SARS-CoV-2 in a Two-Month-Old Infant.Indian J Pediatr. 2023 Jan;90(1):101. doi: 10.1007/s12098-022-04404-9. Epub 2022 Nov 28. Indian J Pediatr. 2023. PMID: 36441385 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous