Daprodustat Compared with Epoetin Beta Pegol for Anemia in Japanese Patients Not on Dialysis: A 52-Week Randomized Open-Label Phase 3 Trial
- PMID: 33561857
- PMCID: PMC8117260
- DOI: 10.1159/000513103
Daprodustat Compared with Epoetin Beta Pegol for Anemia in Japanese Patients Not on Dialysis: A 52-Week Randomized Open-Label Phase 3 Trial
Abstract
Background: Daprodustat is an oral agent that stimulates erythropoiesis by inhibiting the prolyl hydroxylases which mark hypoxia-inducible factor for degradation through hydroxylation. Its safety and efficacy (noninferiority) were assessed in this 52-week, open-label study.
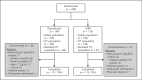
Methods: Japanese patients not on dialysis (ND) (N = 299) with anemia of CKD (stages G3, G4, and G5) with iron parameters of ferritin >100 ng/mL or transferrin saturation >20% at screening were randomized to daprodustat or epoetin beta pegol (continuous erythropoietin receptor activator [CERA], also known as methoxy polyethylene glycol-epoetin beta). After initiation of the study, the daprodustat starting dose for erythropoiesis-stimulating agent (ESA)-naïve participants was revised, and daprodustat was started at 2 or 4 mg once daily depending on baseline hemoglobin. ESA users switched to daprodustat 4 mg once daily. CERA was started at 25 μg every 2 weeks for ESA-naïve patients and 25-250 μg every 4 weeks for ESA users based on previous ESA dose. In both treatment groups, dose was adjusted every 4 weeks based on hemoglobin level and changed according to a prespecified algorithm. The primary endpoint was mean hemoglobin level during weeks 40-52 in the intention-to-treat (ITT) population. ESA-naïve patients who entered before the protocol amendment revising the daprodustat starting dose were excluded from the ITT population.
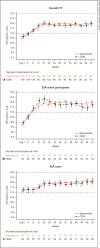
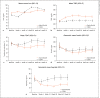
Results: Mean hemoglobin levels during weeks 40-52 were 12.0 g/dL in the daprodustat group (n = 108; 95% confidence interval [CI], 11.8-12.1) and 11.9 g/dL for CERA (n = 109; 95% CI 11.7-12.0); the difference between the groups was 0.1 g/dL (95% CI -0.1 to 0.3 g/dL). The lower limit of the 95% CI of the difference was greater than the prespecified margin of -1.0 g/dL. The mean hemoglobin level was within the target range (11.0-13.0 g/dL) during weeks 40-52 for 92% of participants in both groups. There was no meaningful difference in the frequencies of adverse events.
Conclusions: Oral daprodustat was noninferior to CERA in achieving and maintaining target hemoglobin levels in Japanese ND patients. Daprodustat was well tolerated, with no new safety concerns identified.
Trial registration: ClinicalTrials.gov NCT02791763.
Keywords: Anemia; Daprodustat; Hypoxia-inducible factor; Japanese; Nondialysis.
© 2021 The Author(s) Published by S. Karger AG, Basel.
Conflict of interest statement
M.N. has received grants and personal fees from Astellas Pharma Inc. (Astellas), Chugai Pharmaceutical Co. Ltd. (Chugai), Daiichi Sankyo Co. Ltd., GlaxoSmithKline (GSK), Kyowa Kirin Co. Ltd. (KKC), Mitsubishi Tanabe Pharma, and Torii Pharmaceutical Co. Ltd. (Torii); grants from Bayer Yakuhin Ltd. (Bayer), Ono Pharmaceutical Co. Ltd. (Ono), and Takeda Pharmaceutical Co. Ltd. (Takeda); and personal fees from AstraZeneca and JT Pharmaceuticals. T.H. has received grants and personal fees from Asahi Kasei Pharma Corporation, Bayer, Chugai, Kissei Pharmaceutical Co. Ltd. (Kissei), Otsuka Pharmaceutical Co. Ltd. (Otsuka), and Torii; grants, personal fees, and other from KKC, and Ono; personal fees and other from Astellas; grants from Eisai Co. Ltd., Fuso Pharmaceutical Industries Ltd. (Fuso), Takeda, and Terumo Corporation; and other from GSK. T.A. has received personal fees from Astellas, Bayer, Chugai, Fuso, GSK, JT Pharmaceuticals, KKC, Kissei, Nipro Corporation, Ono, Otsuka, Torii, and Sanwa Chemical Industrial Co. Ltd. Y.T. has received personal fees from Chugai, GSK, KKC, and Torii. R.N. is an employee of GSK. N.O., K.K., T.N., N.P.J., Y.E., and A.R.C. are employees of and hold equity stock in GSK.
Figures
References
-
- Akizawa T, Makino H, Matsuo S, Watanabe T, Imai E, Nitta K, et al. Management of anemia in chronic kidney disease patients: baseline findings from chronic kidney disease Japan cohort study. Clin Exp Nephrol. 2011 Apr;15((2)):248–57. - PubMed
-
- Yamamoto H, Nishi S, Tomo T, Masakane I, Saito K, Nangaku M, et al. 2015 Japanese society for dialysis therapy: guidelines for renal anemia in chronic kidney disease. Ren Replace Ther. 2017 Jun;3((1)):1–46.
-
- Aranesp [package insert] Thousand Oaks, CA: Amgen Inc.; 2019. Jan, Available from: https://www.pi.amgen.com/∼/media/amgen/repositorysites/pi-amgen-com/aran.... Accessed 2019 Apr 4.
-
- Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, et al. Baseline characteristics in the trial to reduce cardiovascular events with aranesp therapy (TREAT) Am J Kidney Dis. 2009 Jul;54((1)):59–69. - PubMed
-
- Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998 Aug;339((9)):584–90. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

