Peritumoral Microenvironment in High-Grade Gliomas: From FLAIRectomy to Microglia-Glioma Cross-Talk
- PMID: 33561993
- PMCID: PMC7915863
- DOI: 10.3390/brainsci11020200
Peritumoral Microenvironment in High-Grade Gliomas: From FLAIRectomy to Microglia-Glioma Cross-Talk
Abstract
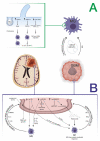
Cellular composition and molecular signatures of the glioma core compared with infiltrative margins are different, and it is well known that the tumor edge is enriched in microglia. In this review of the literature, we summarize the role of the peritumoral area in high-grade gliomas (HGGs) from surgical and biological points of view. There is evidence on the dual role of microglia in HGGs-a scavenger-tumoricidal role when microglia are activated in an M1 phenotype and a role favoring tumor growth and infiltration/migration when microglia are activated in an M2 phenotype. Microglia polarization is mediated by complex pathways involving cross-talk with glioma cells. In this scenario, extracellular vesicles and their miRNA cargo seem to play a central role. The switch to a specific phenotype correlates with prognosis and the pathological assessment of a specific microglial setting can predict a patient's outcome. Some authors have designed an engineered microglial cell as a biologically active vehicle for the delivery of intraoperative near-infrared fluorescent dye with the aim of helping surgeons detect peritumoral infiltrated areas during resection. Furthermore, the pharmacological modulation of microglia-glioma cross-talk paves the way to more effective therapies.
Keywords: 5-ALA; extracellular vesicle; glioblastoma; glioma; immunomodulation; mTOR; microRNA; microglia; supratotal resection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Mampre D., Ehresman J., Pinilla-Monsalve G., Osorio M.A.G., Olivi A., Quinones-Hinojosa A., Chaichana K.L. Extending the resection beyond the contrast-enhancement for glioblastoma: Feasibility, efficacy, and outcomes. Br. J. Neurosurg. 2018;32:528–535. doi: 10.1080/02688697.2018.1498450. - DOI - PubMed
-
- Pessina F., Navarria P., Cozzi L., Ascolese A.M., Simonelli M., Santoro A., Clerici E., Rossi M., Scorsetti M., Bello L. Maximize surgical resection beyond contrast-enhancing boundaries in newly diagnosed glioblastoma multiforme: Is it useful and safe? A single institution retrospective experience. J. Neuro-Oncol. 2017;135:129–139. doi: 10.1007/s11060-017-2559-9. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

