Thermo-Responsive PLGA-PEG-PLGA Hydrogels as Novel Injectable Platforms for Neuroprotective Combined Therapies in the Treatment of Retinal Degenerative Diseases
- PMID: 33562265
- PMCID: PMC7915560
- DOI: 10.3390/pharmaceutics13020234
Thermo-Responsive PLGA-PEG-PLGA Hydrogels as Novel Injectable Platforms for Neuroprotective Combined Therapies in the Treatment of Retinal Degenerative Diseases
Abstract
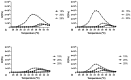
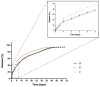
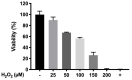
The present study aims to develop a thermo-responsive-injectable hydrogel (HyG) based on PLGA-PEG-PLGA (PLGA = poly-(DL-lactic acid co-glycolic acid); PEG = polyethylene glycol) to deliver neuroprotective agents to the retina over time. Two PLGA-PEG PLGA copolymers with different PEG:LA:GA ratios (1:1.54:23.1 and 1:2.25:22.5) for HyG-1 and HyG-2 development respectively were synthetized and characterized by different techniques (gel permeation chromatography (GPC), nuclear magnetic resonance (NMR), dynamic light scattering (DLS), critical micelle concentration (CMC), gelation and rheological behaviour). According to the physicochemical characterization, HyG-1 was selected for further studies and loaded with anti-inflammatory drugs: dexamethasone (0.2%), and ketorolac (0.5%), alone or in combination with the antioxidants idebenone (1 µM) and D-α-Tocopherol polyethylene glycol 1000 succinate (TPGS) (0.002%). In vitro drug release and cytotoxicity studies were performed for the active substances and hydrogels (loaded and drug-free). A cellular model based on oxidative stress was optimized for anti-inflammatory and antioxidant screening of the formulations by using retinal-pigmented epithelial cell line hTERT (RPE-1). The copolymer 1, used to prepare thermo-responsive HyG-1, showed low polydispersity (PDI = 1.22) and a strong gel behaviour at 25% (w/v) in an isotonic buffer solution close to the vitreous temperature (31-34 °C). Sustained release of dexamethasone and ketorolac was achieved between 47 and 62 days, depending on the composition. HyG-1 was well tolerated (84.5 ± 3.2%) in retinal cells, with values near 100% when the anti-inflammatory and antioxidant agents were included. The combination of idebenone and dexamethasone promoted high oxidative protection in the cells exposed to H2O2, with viability values of 86.2 ± 14.7%. Ketorolac and dexamethasone-based formulations ameliorated the production of TNF-α, showing significant results (p ≤ 0.0001). The hydrogels developed in the present study entail a novel biodegradable tool to treat neurodegenerative processes of the retina overtime.
Keywords: PLGA-PEG-PLGA; dexamethasone; inflammation; intravitreal drug delivery; ketorolac; micelles; neurodegenerative diseases; oxidative stress; thermo-responsive hydrogel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures













References
-
- Flaxman S.R., Bourne R.R.A., Resnikoff S., Ackland P., Braithwaite T., Cicinelli M.V., Das A., Jonas J.B., Keeffe J., Kempen J., et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Health. 2017;5:e1221–e1234. doi: 10.1016/S2214-109X(17)30393-5. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

