The Ins and Outs of RAS Effector Complexes
- PMID: 33562401
- PMCID: PMC7915224
- DOI: 10.3390/biom11020236
The Ins and Outs of RAS Effector Complexes
Abstract
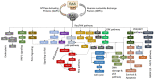
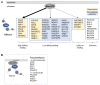
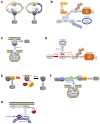
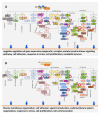
RAS oncogenes are among the most commonly mutated proteins in human cancers. They regulate a wide range of effector pathways that control cell proliferation, survival, differentiation, migration and metabolic status. Including aberrations in these pathways, RAS-dependent signaling is altered in more than half of human cancers. Targeting mutant RAS proteins and their downstream oncogenic signaling pathways has been elusive. However, recent results comprising detailed molecular studies, large scale omics studies and computational modeling have painted a new and more comprehensive portrait of RAS signaling that helps us to understand the intricacies of RAS, how its physiological and pathophysiological functions are regulated, and how we can target them. Here, we review these efforts particularly trying to relate the detailed mechanistic studies with global functional studies. We highlight the importance of computational modeling and data integration to derive an actionable understanding of RAS signaling that will allow us to design new mechanism-based therapies for RAS mutated cancers.
Keywords: RAS in human cancer; RAS oncogene; RAS signaling networks; computational modeling; personalized therapies; targeting RAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

