Portrait of Cancer Stem Cells on Colorectal Cancer: Molecular Biomarkers, Signaling Pathways and miRNAome
- PMID: 33562604
- PMCID: PMC7915330
- DOI: 10.3390/ijms22041603
Portrait of Cancer Stem Cells on Colorectal Cancer: Molecular Biomarkers, Signaling Pathways and miRNAome
Abstract
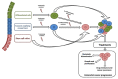
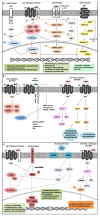
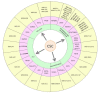
Colorectal cancer (CRC) is a leading cause of cancer death worldwide, and about 20% is metastatic at diagnosis and untreatable. Increasing evidence suggests that the heterogeneous nature of CRC is related to colorectal cancer stem cells (CCSCs), a small cells population with stemness behaviors and responsible for tumor progression, recurrence, and therapy resistance. Growing knowledge of stem cells (SCs) biology has rapidly improved uncovering the molecular mechanisms and possible crosstalk/feedback loops between signaling pathways that directly influence intestinal homeostasis and tumorigenesis. The generation of CCSCs is probably connected to genetic changes in members of signaling pathways, which control self-renewal and pluripotency in SCs and then establish function and phenotype of CCSCs. Particularly, various deregulated CCSC-related miRNAs have been reported to modulate stemness features, controlling CCSCs functions such as regulation of cell cycle genes expression, epithelial-mesenchymal transition, metastasization, and drug-resistance mechanisms. Primarily, CCSC-related miRNAs work by regulating mainly signal pathways known to be involved in CCSCs biology. This review intends to summarize the epigenetic findings linked to miRNAome in the maintenance and regulation of CCSCs, including their relationships with different signaling pathways, which should help to identify specific diagnostic, prognostic, and predictive biomarkers for CRC, but also develop innovative CCSCs-targeted therapies.
Keywords: cancer stem cells; colorectal carcinoma; feedback loop; miRNAs; regulatory network; signaling pathways.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Uleri E., Piu C., Caocci M., Ibba G., Sanges F., Pira G., Murgia L., Barmina M., Giannecchini S., Porcu A., et al. Multiple Signatures of the JC Polyomavirus in Paired Normal and Altered Colorectal Mucosa Indicate a Link with Human Colorectal Cancer, but Not with Cancer Progression. Int. J. Mol. Sci. 2019;20:5965. doi: 10.3390/ijms20235965. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

