Impact of Microbial Metabolites on Microbiota-Gut-Brain Axis in Inflammatory Bowel Disease
- PMID: 33562721
- PMCID: PMC7915037
- DOI: 10.3390/ijms22041623
Impact of Microbial Metabolites on Microbiota-Gut-Brain Axis in Inflammatory Bowel Disease
Abstract
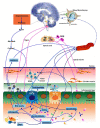
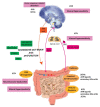
The complex bidirectional communication system existing between the gastrointestinal tract and the brain initially termed the "gut-brain axis" and renamed the "microbiota-gut-brain axis", considering the pivotal role of gut microbiota in sustaining local and systemic homeostasis, has a fundamental role in the pathogenesis of Inflammatory Bowel Disease (IBD). The integration of signals deriving from the host neuronal, immune, and endocrine systems with signals deriving from the microbiota may influence the development of the local inflammatory injury and impacts also more distal brain regions, underlying the psychophysiological vulnerability of IBD patients. Mood disorders and increased response to stress are frequently associated with IBD and may affect the disease recurrence and severity, thus requiring an appropriate therapeutic approach in addition to conventional anti-inflammatory treatments. This review highlights the more recent evidence suggesting that alterations of the microbiota-gut-brain bidirectional communication axis may concur to IBD pathogenesis and sustain the development of both local and CNS symptoms. The participation of the main microbial-derived metabolites, also defined as "postbiotics", such as bile acids, short-chain fatty acids, and tryptophan metabolites in the development of IBD-associated gut and brain dysfunction will be discussed. The last section covers a critical evaluation of the main clinical evidence pointing to the microbiome-based therapeutic approaches for the treatment of IBD-related gastrointestinal and neuropsychiatric symptoms.
Keywords: IBD; antibiotics; dysbiosis; fecal transplant therapy; microbiota targeting therapies; microbiota–gut–brain axis; prebiotics; probiotics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Ng S.C., Shi H.Y., Hamidi N., Underwood F.E., Tang W., Benchimol E.I., Panaccione R., Ghosh S., Wu J.C.Y., Chan F.K.L., et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet. 2017;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

