Effect of Water Microsolvation on the Excited-State Proton Transfer of 3-Hydroxyflavone Enclosed in γ-Cyclodextrin
- PMID: 33562757
- PMCID: PMC7914428
- DOI: 10.3390/molecules26040843
Effect of Water Microsolvation on the Excited-State Proton Transfer of 3-Hydroxyflavone Enclosed in γ-Cyclodextrin
Abstract
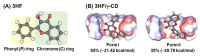
The effect of microsolvation on excited-state proton transfer (ESPT) reaction of 3-hydroxyflavone (3HF) and its inclusion complex with γ-cyclodextrin (γ-CD) was studied using computational approaches. From molecular dynamics simulations, two possible inclusion complexes formed by the chromone ring (C-ring, Form I) and the phenyl ring (P-ring, Form II) of 3HF insertion to γ-CD were observed. Form II is likely more stable because of lower fluctuation of 3HF inside the hydrophobic cavity and lower water accessibility to the encapsulated 3HF. Next, the conformation analysis of these models in the ground (S0) and the first excited (S1) states was carried out by density functional theory (DFT) and time-dependent DFT (TD-DFT) calculations, respectively, to reveal the photophysical properties of 3HF influenced by the γ-CD. The results show that the intermolecular hydrogen bonding (interHB) between 3HF and γ-CD, and intramolecular hydrogen bonding (intraHB) within 3HF are strengthened in the S1 state confirmed by the shorter interHB and intraHB distances and the red-shift of O-H vibrational modes involving in the ESPT process. The simulated absorption and emission spectra are in good agreement with the experimental data. Significantly, in the S1 state, the keto form of 3HF is stabilized by γ-CD, explaining the increased quantum yield of keto emission of 3HF when complexing with γ-CD in the experiment. In the other word, ESPT of 3HF is more favorable in the γ-CD hydrophobic cavity than in aqueous solution.
Keywords: 3-hydroxyflavone (3HF); density functional theory (DFT); excited-state proton transfer (ESPT); molecular dynamics (MD); γ-cyclodextrin (γ-CD).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Uzhinov B.M., Khimich M.N. Conformational effects in excited state intramolecular proton transfer of organic compounds. Russ. Chem. Rev. 2011;80:553–577. doi: 10.1070/RC2011v080n06ABEH004144. - DOI
-
- Weller A. Innermolekularer protonenübergang im angeregten zustand. Z. Elektrochem. Ber. Bunsenges. Phys. Chem. 1956;60:1144–1147.
-
- Goodman J., Brus L.E. Proton transfer and tautomerism in an excited state of methyl salicylate. J. Am. Chem. Soc. 1978;100:7472–7474. doi: 10.1021/ja00492a005. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

