TRPM4 in Cancer-A New Potential Drug Target
- PMID: 33562811
- PMCID: PMC7914809
- DOI: 10.3390/biom11020229
TRPM4 in Cancer-A New Potential Drug Target
Abstract
Transient receptor potential melastatin 4 (TRPM4) is widely expressed in various organs and associated with cardiovascular and immune diseases. Lately, the interest in studies on TRPM4 in cancer has increased. Thus far, TRPM4 has been investigated in diffuse large B-cell lymphoma, prostate, colorectal, liver, breast, urinary bladder, cervical, and endometrial cancer. In several types of cancer TRPM4 is overexpressed and contributes to cancer hallmark functions such as increased proliferation and migration and cell cycle shift. Hence, TRPM4 is a potential prognostic cancer marker and a promising anticancer drug target candidate. Currently, the underlying mechanism by which TRPM4 contributes to cancer hallmark functions is under investigation. TRPM4 is a Ca2+-activated monovalent cation channel, and its ion conductivity can decrease intracellular Ca2+ signaling. Furthermore, TRPM4 can interact with different partner proteins. However, the lack of potent and specific TRPM4 inhibitors has delayed the investigations of TRPM4. In this review, we summarize the potential mechanisms of action and discuss new small molecule TRPM4 inhibitors, as well as the TRPM4 antibody, M4P. Additionally, we provide an overview of TRPM4 in human cancer and discuss TRPM4 as a diagnostic marker and anticancer drug target.
Keywords: calcium; cancer; drug target; ion channel; migration; prognostic marker; proliferation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
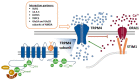
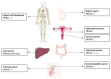
References
-
- Vennekens R., Nilius B. Botulinum Toxin Therapy. Springer Nature; Berlin, Germany: 2007. Insights into TRPM4 Function, Regulation and Physiological Role; pp. 269–285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

