CoronaHiT: high-throughput sequencing of SARS-CoV-2 genomes
- PMID: 33563320
- PMCID: PMC7871948
- DOI: 10.1186/s13073-021-00839-5
CoronaHiT: high-throughput sequencing of SARS-CoV-2 genomes
Abstract
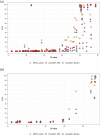
We present CoronaHiT, a platform and throughput flexible method for sequencing SARS-CoV-2 genomes (≤ 96 on MinION or > 96 on Illumina NextSeq) depending on changing requirements experienced during the pandemic. CoronaHiT uses transposase-based library preparation of ARTIC PCR products. Method performance was demonstrated by sequencing 2 plates containing 95 and 59 SARS-CoV-2 genomes on nanopore and Illumina platforms and comparing to the ARTIC LoCost nanopore method. Of the 154 samples sequenced using all 3 methods, ≥ 90% genome coverage was obtained for 64.3% using ARTIC LoCost, 71.4% using CoronaHiT-ONT and 76.6% using CoronaHiT-Illumina, with almost identical clustering on a maximum likelihood tree. This protocol will aid the rapid expansion of SARS-CoV-2 genome sequencing globally.
Keywords: ARTIC; Genetic; Genome; Multiplexing; NGS; Nanopore; SARS-CoV-2; Sequencing.
Conflict of interest statement
LG received a partial support for his PhD from Roche. The use of Roche technology for diagnostics in NNUH is coincidental. The authors declare that they have no competing interests.
Figures


References
-
- COVID-19 Genomics UK Consortium. COVID-19 Genomics UK Consortium [Internet]. [cited 2021 Jan 22]. Available from: https://www.cogconsortium.uk/.
-
- ARTIC Network. ARTIC Network - real-time molecular epidemiology for outbreak response [Internet]. [cited 2021 Jan 22]. Available from: https://artic.network/.
Publication types
MeSH terms
Substances
Grants and funding
- BB/CCG1860/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/R012490/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/F/000PR10356/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/F/000PR10353/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/R012504/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

