Treatment of acute exacerbation of liver-cirrhosis-associated portal vein thrombosis with direct-acting oral anticoagulant, edoxaban, used as an initial treatment in the early postoperative period after abdominal surgery: a case report
- PMID: 33563326
- PMCID: PMC7874483
- DOI: 10.1186/s13256-020-02651-y
Treatment of acute exacerbation of liver-cirrhosis-associated portal vein thrombosis with direct-acting oral anticoagulant, edoxaban, used as an initial treatment in the early postoperative period after abdominal surgery: a case report
Abstract
Background: Cirrhosis-associated portal vein thrombosis (CA-PVT) has been reportedly observed in 5-30% of cirrhotic patients. Moreover, the acute exacerbation of CA-PVT is likely to occur after certain situations, such as a status after abdominal surgery. Safety and efficacy of the direct-acting oral anticoagulant (DOAC) used for cirrhotic patients have been being confirmed. However, use of the DOAC as an initial treatment for CA-PVT appears still challenging especially in the early postoperative period after major surgery in terms of unestablished efficacy and safety in such occasion.
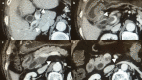
Case presentation: We herein report a case of the acute exacerbation of CA-PVT in the early postoperative period after abdominal surgery, which was successfully treated with DOAC, edoxaban used as an initial treatment. The patient was a 79-year-old Japanese male with alcoholic cirrhosis. The patient suffered choledocholithiasis and had a mural chronic CA-PVT extending from the superior mesenteric vein to the portal trunk. He underwent open cholecystectomy and choledochotomy. Early postoperative clinical course was uneventful except for abdominal distension due to ascites diagnosed on postoperative day (POD)7 when hospital discharge was planned. Contrast enhancement computed tomography (CE-CT) taken on POD 7 revealed the exacerbation of the CA-PVT. Despite recommendation for extension of hospital admission with low molecular weight heparin treatment, the patient strongly hoped to be discharged. Unwillingly, we selected DOAC, edoxaban, as an initial treatment, which was commenced the day after discharge (POD8). As a result, the remarkable improvement of the exacerbated CA-PVT was confirmed by the CE-CT taken on POD21. Any bleeding complications were not observed. Although a slight residue of the CA-PVT remains, the patient is currently doing well 4 years after surgery and is still receiving edoxaban. Any adverse effects of edoxaban have not been observed for 4 years.
Conclusions: A case of successful treatment of the acute exacerbation of CA-PVT with edoxaban was reported. Moreover, edoxaban has been safely administered in a cirrhotic patient for 4 years. The findings obtained from the present case suggest that DOAC can be used as an initial treatment for CA-PVT even in early postoperative period after major abdominal surgery.
Keywords: Direct-acting oral anticoagulant; Early postoperative period after major abdominal surgery; Edoxaban; Liver cirrhosis; Portal vein thrombosis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Hokusai-VTE Investigators, Buller HR, Decousus H, et al. Edoxaban versus warfarin for treatment of symptomatic venous thromboembolism. N Engl J Med 2013;369(15):1406–15. 10.1056/NEJMoa1306638. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

