Evaluating Specimen Quality and Results from a Community-Wide, Home-Based Respiratory Surveillance Study
- PMID: 33563599
- PMCID: PMC8091861
- DOI: 10.1128/JCM.02934-20
Evaluating Specimen Quality and Results from a Community-Wide, Home-Based Respiratory Surveillance Study
Abstract
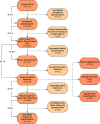
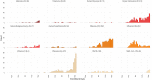
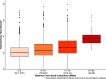
While influenza and other respiratory pathogens cause significant morbidity and mortality, the community-based burden of these infections remains incompletely understood. The development of novel methods to detect respiratory infections is essential for mitigating epidemics and developing pandemic-preparedness infrastructure. From October 2019 to March 2020, we conducted a home-based cross-sectional study in the greater Seattle, WA, area, utilizing electronic consent and data collection instruments. Participants received nasal swab collection kits via rapid delivery within 24 hours of self-reporting respiratory symptoms. Samples were returned to the laboratory and were screened for 26 respiratory pathogens and a housekeeping gene. Participant data were recorded via online survey at the time of sample collection and 1 week later. Of the 4,572 consented participants, 4,359 (95.3%) received a home swab kit and 3,648 (83.7%) returned a nasal specimen for respiratory pathogen screening. The 3,638 testable samples had a mean RNase P relative cycle threshold (Crt ) value of 19.0 (SD, 3.4), and 1,232 (33.9%) samples had positive results for one or more pathogens, including 645 (17.7%) influenza-positive specimens. Among the testable samples, the median time between shipment of the home swab kit and completion of laboratory testing was 8.0 days (interquartile range [IQR], 7.0 to 14.0). A single adverse event occurred and did not cause long-term effects or require medical attention. Home-based surveillance using online participant enrollment and specimen self-collection is a safe and feasible method for community-level monitoring of influenza and other respiratory pathogens, which can readily be adapted for use during pandemics.
Keywords: influenza; nasal swab; pandemic preparedness; rapid diagnosis; respiratory pathogens.
Copyright © 2021 Kim et al.
Figures

References
-
- GBD 2015 LRI Collaborators. 2017. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis 17:1133–1161. 10.1016/S1473-3099(17)30396-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

