NOX1/NADPH Oxidase Promotes Synaptic Facilitation Induced by Repeated D2 Receptor Stimulation: Involvement in Behavioral Repetition
- PMID: 33563722
- PMCID: PMC8018731
- DOI: 10.1523/JNEUROSCI.2121-20.2021
NOX1/NADPH Oxidase Promotes Synaptic Facilitation Induced by Repeated D2 Receptor Stimulation: Involvement in Behavioral Repetition
Abstract
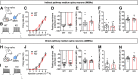
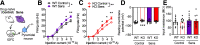
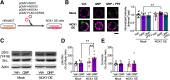
Repetitive behavior is a widely observed neuropsychiatric symptom. Abnormal dopaminergic signaling in the striatum is one of the factors associated with behavioral repetition; however, the molecular mechanisms underlying the induction of repetitive behavior remain unclear. Here, we demonstrated that the NOX1 isoform of the superoxide-producing enzyme NADPH oxidase regulated repetitive behavior in mice by facilitating excitatory synaptic inputs in the central striatum (CS). In male C57Bl/6J mice, repeated stimulation of D2 receptors induced abnormal behavioral repetition and perseverative behavior. Nox1 deficiency or acute pharmacological inhibition of NOX1 significantly shortened repeated D2 receptor stimulation-induced repetitive behavior without affecting motor responses to a single D2 receptor stimulation. Among brain regions, Nox1 showed enriched expression in the striatum, and repeated dopamine D2 receptor stimulation further increased Nox1 expression levels in the CS, but not in the dorsal striatum. Electrophysiological analyses revealed that repeated D2 receptor stimulation facilitated excitatory inputs in the CS indirect pathway medium spiny neurons (iMSNs), and this effect was suppressed by the genetic deletion or pharmacological inhibition of NOX1. Nox1 deficiency potentiated protein tyrosine phosphatase activity and attenuated the accumulation of activated Src kinase, which is required for the synaptic potentiation in CS iMSNs. Inhibition of NOX1 or β-arrestin in the CS was sufficient to ameliorate repetitive behavior. Striatal-specific Nox1 knockdown also ameliorated repetitive and perseverative behavior. Collectively, these results indicate that NOX1 acts as an enhancer of synaptic facilitation in CS iMSNs and plays a key role in the molecular link between abnormal dopamine signaling and behavioral repetition and perseveration.SIGNIFICANCE STATEMENT Behavioral repetition is a form of compulsivity, which is one of the core symptoms of psychiatric disorders, such as obsessive-compulsive disorder. Perseveration is also a hallmark of such disorders. Both clinical and animal studies suggest important roles of abnormal dopaminergic signaling and striatal hyperactivity in compulsivity; however, the precise molecular link between them remains unclear. Here, we demonstrated the contribution of NOX1 to behavioral repetition induced by repeated stimulation of D2 receptors. Repeated stimulation of D2 receptors upregulated Nox1 mRNA in a striatal subregion-specific manner. The upregulated NOX1 promoted striatal synaptic facilitation in iMSNs by enhancing phosphorylation signaling. These results provide a novel mechanism for D2 receptor-mediated excitatory synaptic facilitation and indicate the therapeutic potential of NOX1 inhibition in compulsivity.
Keywords: NADPH oxidase; behavioral repetition; dopamine; obsessive-compulsive disorder; striatum.
Copyright © 2021 the authors.
Figures








References
-
- Arakawa N, Katsuyama M, Matsuno K, Urao N, Tabuchi Y, Okigaki M, Matsubara H, Yabe-Nishimura C (2006) Novel transcripts of Nox1 are regulated by alternative promoters and expressed under phenotypic modulation of vascular smooth muscle cells. Biochem J 398:303–310. 10.1042/BJ20060300 - DOI - PMC - PubMed
-
- Bailey A, Metaxas A, Yoo JH, McGee T, Kitchen I (2008) Decrease of D2 receptor binding but increase in D2-stimulated G-protein activation, dopamine transporter binding and behavioural sensitization in brains of mice treated with a chronic escalating dose 'binge' cocaine administration paradigm. Eur J Neurosci 28:759–770. 10.1111/j.1460-9568.2008.06369.x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
